IPC Semester Exam Review – Chemistry Topics
... How many centimeters long is the proverbial “10-foot pole?” 18. How many milliliters are in a 2.0 quart jug of milk? 19. Calculate density from the slope of a graph (see lab report). VOCAB: number, quantity 33. When atmospheric pressure increases, boiling point __. 34. Describe energy and particles ...
... How many centimeters long is the proverbial “10-foot pole?” 18. How many milliliters are in a 2.0 quart jug of milk? 19. Calculate density from the slope of a graph (see lab report). VOCAB: number, quantity 33. When atmospheric pressure increases, boiling point __. 34. Describe energy and particles ...
x - A Level Tuition
... Either a burette with 0.1 cm3 interval or a measuring cylinder with 1 cm3 interval can be used to measure the volume of FA 3 required. It is known that the error (or uncertainty) that is associated with each reading when using a measuring cylinder with 1 cm3 interval is ±0.5 cm3, while that using a ...
... Either a burette with 0.1 cm3 interval or a measuring cylinder with 1 cm3 interval can be used to measure the volume of FA 3 required. It is known that the error (or uncertainty) that is associated with each reading when using a measuring cylinder with 1 cm3 interval is ±0.5 cm3, while that using a ...
CHEMISTRY 1710 - Practice Exam #2 (KATZ)
... A) H+(aq) + OH-(aq) → H2O(l) B) 2 K+(aq) + SO42-(aq) → K2SO4(s) C) H+(aq) + OH-(aq) + 2 K+(aq) + SO42-(aq) → H2O(l) + K2SO4(s) D) H22+(aq) + OH-(aq) → H2(OH)2(l) _____ 18. The titration of 25.0 mL of an unknown concentration H2SO4 solution requires 83.6 mL of 0.12 M LiOH solution. What is the concen ...
... A) H+(aq) + OH-(aq) → H2O(l) B) 2 K+(aq) + SO42-(aq) → K2SO4(s) C) H+(aq) + OH-(aq) + 2 K+(aq) + SO42-(aq) → H2O(l) + K2SO4(s) D) H22+(aq) + OH-(aq) → H2(OH)2(l) _____ 18. The titration of 25.0 mL of an unknown concentration H2SO4 solution requires 83.6 mL of 0.12 M LiOH solution. What is the concen ...
qp13 - Smart Edu Hub
... 32 A new planet has been discovered and its atmosphere has been analysed. atmosphere ...
... 32 A new planet has been discovered and its atmosphere has been analysed. atmosphere ...
Lecture 4
... 4. Cancel the spectator ions on both sides of the ionic equation Write the net ionic equation for the reaction of silver nitrate with sodium chloride. ...
... 4. Cancel the spectator ions on both sides of the ionic equation Write the net ionic equation for the reaction of silver nitrate with sodium chloride. ...
Atoms, molecules and ions
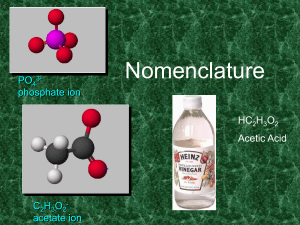
... • For an oxyacid (general formula HmXOn) it often happens that there are multiple possible values of n for each element X, and as such, within this series of compounds, – There is always an acid in the series that ends with “ic” • Adding another oxygen to the “ic” acid produces the “per….ic” acid • ...
... • For an oxyacid (general formula HmXOn) it often happens that there are multiple possible values of n for each element X, and as such, within this series of compounds, – There is always an acid in the series that ends with “ic” • Adding another oxygen to the “ic” acid produces the “per….ic” acid • ...
Acid rain
Acid rain is a rain or any other form of precipitation that is unusually acidic, meaning that it possesses elevated levels of hydrogen ions (low pH). It can have harmful effects on plants, aquatic animals and infrastructure. Acid rain is caused by emissions of sulfur dioxide and nitrogen oxide, which react with the water molecules in the atmosphere to produce acids. Governments have made efforts since the 1970s to reduce the release of sulfur dioxide into the atmosphere with positive results. Nitrogen oxides can also be produced naturally by lightning strikes and sulfur dioxide is produced by volcanic eruptions. The chemicals in acid rain can cause paint to peel, corrosion of steel structures such as bridges, and erosion of stone statues.