
Chapter 4 Stoichiometry Power Point
... Many reactions release a gaseous product. Although a wide variety of these gasforming reactions occur, some of the most important gases produced in reactions are the following: Acid/Base Rxns that form Gases: S2- (Sulfides) and CO32-(Carbonate), HCO3(Bicarbonate) ions react with acids to form gases: ...
... Many reactions release a gaseous product. Although a wide variety of these gasforming reactions occur, some of the most important gases produced in reactions are the following: Acid/Base Rxns that form Gases: S2- (Sulfides) and CO32-(Carbonate), HCO3(Bicarbonate) ions react with acids to form gases: ...
WRITING AP EQUATIONS AP equation sets are found in the free
... General guidelines (may not always be true!): One point is given for the correct reactants and two points for all correct products. If a reaction has three products, one point is given for two correct products and two points for all correct products. One point will be given for correct balancing and ...
... General guidelines (may not always be true!): One point is given for the correct reactants and two points for all correct products. If a reaction has three products, one point is given for two correct products and two points for all correct products. One point will be given for correct balancing and ...
ATOMS, MOLECULES, AND IONS
... a given compound. (e.g. water, H2 O, always contains 2 hydrogen atoms and 1 oxygen atom) The Law of Conservation of Mass — during a chemical reaction, the total mass before reaction is equal to the total mass after reaction. N2 + 3 H 2 → 2 NH 3 ...
... a given compound. (e.g. water, H2 O, always contains 2 hydrogen atoms and 1 oxygen atom) The Law of Conservation of Mass — during a chemical reaction, the total mass before reaction is equal to the total mass after reaction. N2 + 3 H 2 → 2 NH 3 ...
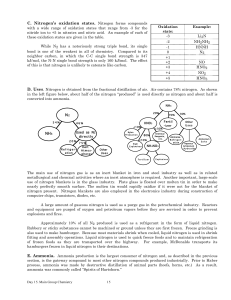
Nitrogen`s oxidation states
... and N2O5 . The trademark of their chemistry is their ability to interconvert so it is difficult to study any one pure oxide. All of these oxides are acid anhydrides. Nitrous oxide, N2O. The proper IUPAC name N2O is dinitrogen monoxide, however its common name, nitrous oxide, is widely used. It is al ...
... and N2O5 . The trademark of their chemistry is their ability to interconvert so it is difficult to study any one pure oxide. All of these oxides are acid anhydrides. Nitrous oxide, N2O. The proper IUPAC name N2O is dinitrogen monoxide, however its common name, nitrous oxide, is widely used. It is al ...
Safety Data Sheet - Fisher Scientific
... appropriate fire suppression agents for adjacent combustible materials or sources of ignition. Use dry Chemical, foam, or carbon dioxide to extinguish fire. For safety reasons unsuitable extinguishing agents: Do not use water directly on sulfuric acid Special hazards arising from the substance or mi ...
... appropriate fire suppression agents for adjacent combustible materials or sources of ignition. Use dry Chemical, foam, or carbon dioxide to extinguish fire. For safety reasons unsuitable extinguishing agents: Do not use water directly on sulfuric acid Special hazards arising from the substance or mi ...
Chem 150 - Fall 2015 Exam I
... Element symbols and names: symbols, names, and spellings are recommended by IUPAC (http://www.iupac.org/). Names are not yet proposed for the elements beyond 111 - those used here are IUPAC’s temporary systematic names (Pure & Appl. Chem., 1979, 51, 381–384). In the USA and some other countries, the ...
... Element symbols and names: symbols, names, and spellings are recommended by IUPAC (http://www.iupac.org/). Names are not yet proposed for the elements beyond 111 - those used here are IUPAC’s temporary systematic names (Pure & Appl. Chem., 1979, 51, 381–384). In the USA and some other countries, the ...
2nd Semester Final Exam Review
... 27. The melting of 1 mole of H2O takes 1.44 kcal of energy. Calculate the energy involved if only 3.15 grams of ice were melted. q= H x n 28. In the problem above did the entropy increase, decrease, or not change? Explain. 29. If 45.0 g of water is heated and the temp. rose from 20.6 oC to 30.0 oC. ...
... 27. The melting of 1 mole of H2O takes 1.44 kcal of energy. Calculate the energy involved if only 3.15 grams of ice were melted. q= H x n 28. In the problem above did the entropy increase, decrease, or not change? Explain. 29. If 45.0 g of water is heated and the temp. rose from 20.6 oC to 30.0 oC. ...
2018 Specimen Paper 2 - Cambridge International Examinations
... What additional apparatus is required to identify the amino acids present in a mixture? ...
... What additional apparatus is required to identify the amino acids present in a mixture? ...
H 2 SO 4
... Many reactions release a gaseous product. Although a wide variety of these gasforming reactions occur, some of the most important gases produced in reactions are the following: Acid/Base Rxns that form Gases: S2- (Sulfides) and CO32-(Carbonate), HCO3(Bicarbonate) ions react with acids to form gases: ...
... Many reactions release a gaseous product. Although a wide variety of these gasforming reactions occur, some of the most important gases produced in reactions are the following: Acid/Base Rxns that form Gases: S2- (Sulfides) and CO32-(Carbonate), HCO3(Bicarbonate) ions react with acids to form gases: ...
A.P. Chemistry
... Problem: What volume of 16 M sulfuric acid must be used to prepare 1.5 L of a 0.10 M H2SO4 solution? ...
... Problem: What volume of 16 M sulfuric acid must be used to prepare 1.5 L of a 0.10 M H2SO4 solution? ...
Acid rain
Acid rain is a rain or any other form of precipitation that is unusually acidic, meaning that it possesses elevated levels of hydrogen ions (low pH). It can have harmful effects on plants, aquatic animals and infrastructure. Acid rain is caused by emissions of sulfur dioxide and nitrogen oxide, which react with the water molecules in the atmosphere to produce acids. Governments have made efforts since the 1970s to reduce the release of sulfur dioxide into the atmosphere with positive results. Nitrogen oxides can also be produced naturally by lightning strikes and sulfur dioxide is produced by volcanic eruptions. The chemicals in acid rain can cause paint to peel, corrosion of steel structures such as bridges, and erosion of stone statues.






![[edit]Occurrence in solution](http://s1.studyres.com/store/data/009755146_1-58e56f0cc08d3d020872dbc6c3acbb66-300x300.png)















