Experiment 1 - 8. Form of Energy
... horizontal axis, which shows a straight line in agreement with equation (4). Its slope is 103 q/P and it meets the vertical axis (m=0) at qC/P. Hence we can obtain the values of the mechanical equivalent of heat c and the heat capacity of calorimeter C. ...
... horizontal axis, which shows a straight line in agreement with equation (4). Its slope is 103 q/P and it meets the vertical axis (m=0) at qC/P. Hence we can obtain the values of the mechanical equivalent of heat c and the heat capacity of calorimeter C. ...
Manual(Exp.1)
... m is the horizontal axis, which shows a straight line in agreement with equation (4). Its slope is 103 q/P and it meets the vertical axis (m=0) at qC/P. Hence we can obtain the values of the mechanical equivalent of heat c and the heat capacity of calorimeter C. Although the calorimeter was designed ...
... m is the horizontal axis, which shows a straight line in agreement with equation (4). Its slope is 103 q/P and it meets the vertical axis (m=0) at qC/P. Hence we can obtain the values of the mechanical equivalent of heat c and the heat capacity of calorimeter C. Although the calorimeter was designed ...
Thermodynamics
... In thermodynamics, “system” still means that. However, we add the notion that the system will usually include some definite amount of a fluid – typically, an ideal gas. It might also include other elements, such as the fluid’s container. It’s always important to be clear about what’s in the system, ...
... In thermodynamics, “system” still means that. However, we add the notion that the system will usually include some definite amount of a fluid – typically, an ideal gas. It might also include other elements, such as the fluid’s container. It’s always important to be clear about what’s in the system, ...
Thermal properties Heat capacity C = ΔQ/ΔT = dQ/dT [J/deg] Heat
... (discussed in Chapter 18) also affect thermal conductivity in metals. E.g., adding impurities introduces scattering centers for conduction band electrons and reduce k. ...
... (discussed in Chapter 18) also affect thermal conductivity in metals. E.g., adding impurities introduces scattering centers for conduction band electrons and reduce k. ...
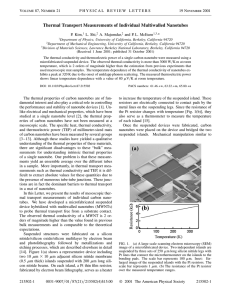
Thermal Transport Measurements of Individual Multiwalled Nanotubes
... the intrinsic axial thermal conductivity of a MWNT. Further study to analyze the contribution of individual layers of MWNTs [19] in the thermal transport should elucidate this important issue in the future. Shown in the lower inset of Fig. 3 is the temperature dependent thermal conductivity, k共T兲 (s ...
... the intrinsic axial thermal conductivity of a MWNT. Further study to analyze the contribution of individual layers of MWNTs [19] in the thermal transport should elucidate this important issue in the future. Shown in the lower inset of Fig. 3 is the temperature dependent thermal conductivity, k共T兲 (s ...
Chapter 6
... Identify each energy change as primarily heat or work, and determine whether Esys is positive or negative. a. One billiard ball (the system) hits another one, and stops rolling. b. A book (the system) is dropped on the floor c. A father pushes his daughter on the swing (the daughter & swing are the ...
... Identify each energy change as primarily heat or work, and determine whether Esys is positive or negative. a. One billiard ball (the system) hits another one, and stops rolling. b. A book (the system) is dropped on the floor c. A father pushes his daughter on the swing (the daughter & swing are the ...
Chapter 1 Thermodynamics
... computer may work; for N ≥ 1googol = 10100 statistical physics may be the only tool. There are two standard ways to study the large N limit: • phenomenological (e.g. thermodynamics) and • fundamental (e.g. statistical mechanics). ...
... computer may work; for N ≥ 1googol = 10100 statistical physics may be the only tool. There are two standard ways to study the large N limit: • phenomenological (e.g. thermodynamics) and • fundamental (e.g. statistical mechanics). ...
heat engine
... An ideal, or Carnot, heat pump is used to heat a house at 294 K. How much work must the pump do to deliver 3350 J of heat into the house on a day when the outdoor temperature is 273 K? ...
... An ideal, or Carnot, heat pump is used to heat a house at 294 K. How much work must the pump do to deliver 3350 J of heat into the house on a day when the outdoor temperature is 273 K? ...
PPT
... No process is possible whose sole result is the transfer of heat from a body of lower temperature to a body of higher temperature The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated ...
... No process is possible whose sole result is the transfer of heat from a body of lower temperature to a body of higher temperature The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated ...
Chapter 6
... motion whether it deals with acoustic waves, spiral arms in hurricanes, weather waves in the atmosphere or the meandering Gulf Stream in the ocean. This very richness in the basic equations is an impediment to solving any one of those examples since for some phenomenon of interest we have included ...
... motion whether it deals with acoustic waves, spiral arms in hurricanes, weather waves in the atmosphere or the meandering Gulf Stream in the ocean. This very richness in the basic equations is an impediment to solving any one of those examples since for some phenomenon of interest we have included ...
B.Sc. Program Phys Courses (English)
... Light: Reflection, refraction, elimination, image formation, the lens equation, magnification, the telescope, spherical mirrors. ...
... Light: Reflection, refraction, elimination, image formation, the lens equation, magnification, the telescope, spherical mirrors. ...
B.Sc. Program Phys Courses (English)
... Light: Reflection, refraction, elimination, image formation, the lens equation, magnification, the telescope, spherical mirrors. ...
... Light: Reflection, refraction, elimination, image formation, the lens equation, magnification, the telescope, spherical mirrors. ...
INTRODUCTION - WordPress.com
... instruments. In this approach, the structure of matter is not considered and no attention is focused on the behavior of the individual particles constituting the matter. The study is made of overall effect of several molecules; the behavior and activities of the molecules are averaged, i.e., their e ...
... instruments. In this approach, the structure of matter is not considered and no attention is focused on the behavior of the individual particles constituting the matter. The study is made of overall effect of several molecules; the behavior and activities of the molecules are averaged, i.e., their e ...
Document
... Example 19-10: First law in isobaric and isovolumetric processes. An ideal gas is slowly compressed at a constant pressure of 2.0 atm from 10.0 L to 2.0 L. (In this process, some heat flows out of the gas and the temperature drops.) Heat is then added to the gas, holding the volume constant, and the ...
... Example 19-10: First law in isobaric and isovolumetric processes. An ideal gas is slowly compressed at a constant pressure of 2.0 atm from 10.0 L to 2.0 L. (In this process, some heat flows out of the gas and the temperature drops.) Heat is then added to the gas, holding the volume constant, and the ...
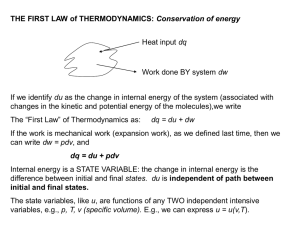
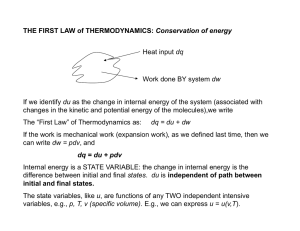
THE FIRST LAW of THERMODYNAMICS: Conservation of energy
... The Adiabatic Air Parcel Idealized model of an air parcel is a small volume of air: • Thermally insulated from the environment so temperature changes are adiabatic • Parcel pressure immediately adjusts to environmental pressure • Parcel moves slowly enough such that macroscopic KE is negligible ...
... The Adiabatic Air Parcel Idealized model of an air parcel is a small volume of air: • Thermally insulated from the environment so temperature changes are adiabatic • Parcel pressure immediately adjusts to environmental pressure • Parcel moves slowly enough such that macroscopic KE is negligible ...
Thermal radiation
Thermal radiation is electromagnetic radiation generated by the thermal motion of charged particles in matter. An object with a temperature greater than absolute zero emits thermal radiation. When the temperature of the body is greater than absolute zero, interatomic collisions cause the kinetic energy of the atoms or molecules to change. This results in charge-acceleration and/or dipole oscillation which produces electromagnetic radiation, and the wide spectrum of radiation reflects the wide spectrum of energies and accelerations that occur even at a single temperature.Examples of thermal radiation include the visible light and infrared light emitted by an incandescent light bulb, the infrared radiation emitted by animals and detectable with an infrared camera, and the cosmic microwave background radiation. Thermal radiation is different from thermal convection and thermal conduction—a person near a raging bonfire feels radiant heating from the fire, even if the surrounding air is very cold.Sunlight is part of thermal radiation generated by the hot plasma of the Sun. The Earth also emits thermal radiation, but at a much lower intensity and different spectral distribution (infrared rather than visible) because it is cooler. The Earth's absorption of solar radiation, followed by its outgoing thermal radiation are the two most important processes that determine the temperature and climate of the Earth.If a radiation-emitting object meets the physical characteristics of a black body in thermodynamic equilibrium, the radiation is called blackbody radiation. Planck's law describes the spectrum of blackbody radiation, which depends only on the object's temperature. Wien's displacement law determines the most likely frequency of the emitted radiation, and the Stefan–Boltzmann law gives the radiant intensity.Thermal radiation is one of the fundamental mechanisms of heat transfer.






![Thermal properties Heat capacity C = ΔQ/ΔT = dQ/dT [J/deg] Heat](http://s1.studyres.com/store/data/015132718_1-30af002d7b96997c56f474559859d37b-300x300.png)