Unit 1 Student Booklet
... reactant and product. 2. Write each element underneath the equation and keep a tally of the number of atoms of each element. 3. Use coefficients to balance metals first, then non-metals. 4. Leave single elements and diatomic molecules to balance last. 5. If possible, reduce the coefficients to the l ...
... reactant and product. 2. Write each element underneath the equation and keep a tally of the number of atoms of each element. 3. Use coefficients to balance metals first, then non-metals. 4. Leave single elements and diatomic molecules to balance last. 5. If possible, reduce the coefficients to the l ...
Belarus, National Final, 2001 (PDF 149K).
... creased by acidifying the solution (using, for example, nitric acid). a) Calculate the molar concentration of silver acetate in a solution saturated at 20o C, if the density of the solution is 1.01 g/cm3. b) Calculate the solubility product constant for silver acetate. c) What is the pH of a solutio ...
... creased by acidifying the solution (using, for example, nitric acid). a) Calculate the molar concentration of silver acetate in a solution saturated at 20o C, if the density of the solution is 1.01 g/cm3. b) Calculate the solubility product constant for silver acetate. c) What is the pH of a solutio ...
PDF of this page
... Laboratory experiments supplement the study of the listed topics. CHEM 1151K. Survey of Chemistry I. 4 Hours. A study of the fundamental principles of chemistry emphasizing modern atomic theory, the structure and behavior of atoms, the properties and states of matter, energy relations, periodicity a ...
... Laboratory experiments supplement the study of the listed topics. CHEM 1151K. Survey of Chemistry I. 4 Hours. A study of the fundamental principles of chemistry emphasizing modern atomic theory, the structure and behavior of atoms, the properties and states of matter, energy relations, periodicity a ...
Stoichiometry – AP - Waukee Community School District Blogs
... Aqueous solutions of sodium chloride and silver nitrate react to produce aqueous sodium nitrate and a precipitate of silver chloride. Bubbling chlorine gas through a solution of potassium iodide gives elemental iodine and a solution of potassium chloride. ...
... Aqueous solutions of sodium chloride and silver nitrate react to produce aqueous sodium nitrate and a precipitate of silver chloride. Bubbling chlorine gas through a solution of potassium iodide gives elemental iodine and a solution of potassium chloride. ...
The Chemistry of Burgers
... ROKER: That is, in fresh, raw meat. Meat changes color, in a series of chemical reactions, when you apply heat: Put a burger on a grill or in a skillet, and it turns brown because of what happens to the myoglobin as you’re about to see. Julie Yu is an NSF-funded chemist at The Exploratorium in San F ...
... ROKER: That is, in fresh, raw meat. Meat changes color, in a series of chemical reactions, when you apply heat: Put a burger on a grill or in a skillet, and it turns brown because of what happens to the myoglobin as you’re about to see. Julie Yu is an NSF-funded chemist at The Exploratorium in San F ...
PSI AP Chemistry Name Unit 4: Chemical Bonding MC Review Part
... 78. The liquefied hydrogen halides have the normal boiling points given below. The relatively high boiling point of HF can be correctly explained by which of the following? (A) HF gas is more ideal. (B) HF is the strongest acid. (C) HF molecules have a smaller dipole moment. (D) HF is much less solu ...
... 78. The liquefied hydrogen halides have the normal boiling points given below. The relatively high boiling point of HF can be correctly explained by which of the following? (A) HF gas is more ideal. (B) HF is the strongest acid. (C) HF molecules have a smaller dipole moment. (D) HF is much less solu ...
WORD - SSS Chemistry
... Make the following conversions, clearly showing your steps. Include proper units in all of your work and in your answer. a) ...
... Make the following conversions, clearly showing your steps. Include proper units in all of your work and in your answer. a) ...
CPGAN #021 Determination of ash content in plastics Summary This
... chemical components of the ash without additional test procedures being performed, such as FTIR analysis or TGA. When FTIR is combined with TGA, the individual components of the ash residual can be identified. Procedure The calcination method is used to determine the ash residual in two forms of pla ...
... chemical components of the ash without additional test procedures being performed, such as FTIR analysis or TGA. When FTIR is combined with TGA, the individual components of the ash residual can be identified. Procedure The calcination method is used to determine the ash residual in two forms of pla ...
Mass Spectrometry and Organic
... •Find MW by other method Other compounds present may give ions that deceive us. May be more detectable. •Prepare derivative MS intensities are problematic ...
... •Find MW by other method Other compounds present may give ions that deceive us. May be more detectable. •Prepare derivative MS intensities are problematic ...
June 2000 Practice Diploma
... release approximately 22.6 kJ of energy release approximately 40.8 kJ of energy absorb approximately 22.6 kJ of energy absorb approximately 40.8 kJ of energy ...
... release approximately 22.6 kJ of energy release approximately 40.8 kJ of energy absorb approximately 22.6 kJ of energy absorb approximately 40.8 kJ of energy ...
Chemistry - talcher autonomous college
... Ionic equilibria - II: Salt hydrolysis-calculation of hydrolysis constant, degree of hydrolysis and pH for different salts. Buffer solutions; derivation of Henderson equation and its applications; buffer capacity, buffer range, buffer action and applications of buffers in analytical chemistry and bi ...
... Ionic equilibria - II: Salt hydrolysis-calculation of hydrolysis constant, degree of hydrolysis and pH for different salts. Buffer solutions; derivation of Henderson equation and its applications; buffer capacity, buffer range, buffer action and applications of buffers in analytical chemistry and bi ...
Lab 1-1 - My eCoach
... INTRODUCTION: Chemistry is a science that investigates changes in matter. Chemical reactions are the changes matter undergoes. The changes you can observe are called “macroscopic changes.” Often these changes, such as color changes, the formation of a solid (precipitation), or the formation of gas b ...
... INTRODUCTION: Chemistry is a science that investigates changes in matter. Chemical reactions are the changes matter undergoes. The changes you can observe are called “macroscopic changes.” Often these changes, such as color changes, the formation of a solid (precipitation), or the formation of gas b ...
Chemistry
... JOHN MCMURRY, educated at Harvard and Columbia, has taught approximately 17,000 students in general and organic chemistry over a 30-year period. A Professor of Chemistry at Cornell University since 1980, Dr. McMurry previously spent 13 years on the faculty at the University of California at Santa Cr ...
... JOHN MCMURRY, educated at Harvard and Columbia, has taught approximately 17,000 students in general and organic chemistry over a 30-year period. A Professor of Chemistry at Cornell University since 1980, Dr. McMurry previously spent 13 years on the faculty at the University of California at Santa Cr ...
Separation and Purification Methods
... moving (thus the name), then the more time a compound spends in that phase, the farther it will travel. Chromatographic techniques fall into one of two categories: analytical and preparative. Analytical techniques are used to follow the course of reactions and determine purity of products. These met ...
... moving (thus the name), then the more time a compound spends in that phase, the farther it will travel. Chromatographic techniques fall into one of two categories: analytical and preparative. Analytical techniques are used to follow the course of reactions and determine purity of products. These met ...
Know (main topic)
... - -memorize the 3 subatomic particles of an atom -determine atomic number, mass number, protons, electrons, & neutrons from the periodic table -explain why the nucleus of the atom is much smaller than the atom yet contains most of its mass -relate the position of an element in the Periodic Table to ...
... - -memorize the 3 subatomic particles of an atom -determine atomic number, mass number, protons, electrons, & neutrons from the periodic table -explain why the nucleus of the atom is much smaller than the atom yet contains most of its mass -relate the position of an element in the Periodic Table to ...
A millennial overview of transition metal chemistry
... often useful magnetic properties.2 These magnetic properties range from simple Curie paramagnetism to those associated with high-temperature superconductivity. At the beginning of the first millennium (i.e., six days after the birthday arbitrarily assumed for Jesus) only the following transition elem ...
... often useful magnetic properties.2 These magnetic properties range from simple Curie paramagnetism to those associated with high-temperature superconductivity. At the beginning of the first millennium (i.e., six days after the birthday arbitrarily assumed for Jesus) only the following transition elem ...
Physical Chemistry: Thermodynamics, Structure, And Change By
... 0716787598 - Physical Chemistry by Atkins, Peter; Physical Chemistry by Atkins, Peter; de Paula, Physical Chemistry. Peter Atkins; Julio de Paula. Phase diagrams 7. Chemical equilibrium PART 2: STRUCTURE 8. OUP: Berry: Physical Chemistry - Oxford University Physical Chemistry is a textbook for cours ...
... 0716787598 - Physical Chemistry by Atkins, Peter; Physical Chemistry by Atkins, Peter; de Paula, Physical Chemistry. Peter Atkins; Julio de Paula. Phase diagrams 7. Chemical equilibrium PART 2: STRUCTURE 8. OUP: Berry: Physical Chemistry - Oxford University Physical Chemistry is a textbook for cours ...
CERAMICS MATERIALS - Wits Structural Chemistry
... are synthesis methods used in materials chemistry. Temperatures used in solution methods are lower than in direct methods and low temperature may also be used to reduce the size of particles of materials prepared. ...
... are synthesis methods used in materials chemistry. Temperatures used in solution methods are lower than in direct methods and low temperature may also be used to reduce the size of particles of materials prepared. ...
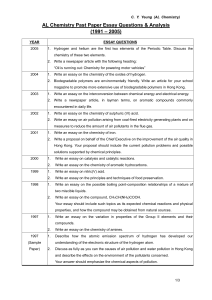
AL Chemistry Past paper essay questions
... Write an essay on amino acids, polypeptides and proteins. Your essay should include the properties of amino acids in aqueous solutions and a method of separation for a mixture of amino acids, as well as the constitution of polypeptides and proteins and their hydrolysis. ...
... Write an essay on amino acids, polypeptides and proteins. Your essay should include the properties of amino acids in aqueous solutions and a method of separation for a mixture of amino acids, as well as the constitution of polypeptides and proteins and their hydrolysis. ...
A LIFE SCIENTIST`S GUIDE TO PHYSICAL CHEMISTRY
... to set some time aside every week to read the book and to solve some problems. Reading a physical chemistry book is something you do with a pad of paper, a pencil and a calculator, because you usually need to work out some of the steps in the derivations or in the examples in order to make sure you ...
... to set some time aside every week to read the book and to solve some problems. Reading a physical chemistry book is something you do with a pad of paper, a pencil and a calculator, because you usually need to work out some of the steps in the derivations or in the examples in order to make sure you ...
B.Sc. Physical Sciences - Department of Computer Science
... Sequences to be introduced through the examples arising in Science beginning with finite sequences, followed by concepts of recursion and difference equations. For instance, the sequence arising from Tower of Hanoi game, the Fibonacci sequence arising from branching habit of trees and breeding habit ...
... Sequences to be introduced through the examples arising in Science beginning with finite sequences, followed by concepts of recursion and difference equations. For instance, the sequence arising from Tower of Hanoi game, the Fibonacci sequence arising from branching habit of trees and breeding habit ...
Chemistry B2A Chapter 18 Oxidation
... In some reactions, it is not easy to see the electron loss and gain, so chemists developed another definition of oxidation and reduction: Oxidation is the gain of oxygen atoms and/or the loss of hydrogen atoms. Reduction is the loss of oxygen atoms and/or the gain of hydrogen atoms. CH4(g) + 2O2(g) ...
... In some reactions, it is not easy to see the electron loss and gain, so chemists developed another definition of oxidation and reduction: Oxidation is the gain of oxygen atoms and/or the loss of hydrogen atoms. Reduction is the loss of oxygen atoms and/or the gain of hydrogen atoms. CH4(g) + 2O2(g) ...
B.Sc. Physical Sciences - Educational Multimedia Research Centre
... Differentiation of a vector with respect to a scalar. Gradient, divergence, curl and Laplacian operations and their meanings. Idea of line, surface and volume integrals. Gauss’s divergence theorem, Stokes theorem and Greens’s theorem in a Plane. Mechanics (Total Number of Lectures = 30) Dynamics of ...
... Differentiation of a vector with respect to a scalar. Gradient, divergence, curl and Laplacian operations and their meanings. Idea of line, surface and volume integrals. Gauss’s divergence theorem, Stokes theorem and Greens’s theorem in a Plane. Mechanics (Total Number of Lectures = 30) Dynamics of ...
Analytical chemistry

Analytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample, and quantitative analysis determines the amount of certain components in the substance. The separation of components is often performed prior to analysis.Analytical methods can be separated into classical and instrumental. Classical methods (also known as wet chemistry methods) use separations such as precipitation, extraction, and distillation and qualitative analysis by color, odor, or melting point. Classical quantitative analysis is achieved by measurement of weight or volume. Instrumental methods use an apparatus to measure physical quantities of the analyte such as light absorption, fluorescence, or conductivity. The separation of materials is accomplished using chromatography, electrophoresis or field flow fractionation methods.Analytical chemistry is also focused on improvements in experimental design, chemometrics, and the creation of new measurement tools to provide better chemical information. Analytical chemistry has applications in forensics, bioanalysis, clinical analysis, environmental analysis, and materials analysis.