Chemistry - NIC Karnataka
... + SO3(aq) Cr 3aq + SO24(aq) (acidic medium) Cr2O7(aq) MnO4–(aq) + Br–(aq) MnO2(s)+BrO3–(aq)(acidic medium) b) Half reaction method : Fe2+(aq)+Cr2O72–(aq) Fe3+(aq)+Cr3+aq) (acidic medium) MnO4–(aq) + I–(aq) MnO2(s) + I2(s) (basic medium) Applications: redox titrations, redox indicators - with example ...
... + SO3(aq) Cr 3aq + SO24(aq) (acidic medium) Cr2O7(aq) MnO4–(aq) + Br–(aq) MnO2(s)+BrO3–(aq)(acidic medium) b) Half reaction method : Fe2+(aq)+Cr2O72–(aq) Fe3+(aq)+Cr3+aq) (acidic medium) MnO4–(aq) + I–(aq) MnO2(s) + I2(s) (basic medium) Applications: redox titrations, redox indicators - with example ...
Possible pieces of introduction:
... idea as a yearning to connect to people through chemistry when he finds himself alone in Milan, forced to perform a job he does not believe in. He states “I felt a widower and an orphan and fantasized about writing the saga of an atom of carbon, to make the people understand the solemn poetry, known ...
... idea as a yearning to connect to people through chemistry when he finds himself alone in Milan, forced to perform a job he does not believe in. He states “I felt a widower and an orphan and fantasized about writing the saga of an atom of carbon, to make the people understand the solemn poetry, known ...
James Moir as Inorganic Chemist
... The structures of Ag(tu)2Cl and Au(tu)2Br have also been determined and shown to be similarly complex compounds.13,14 Moir succeeded in isolating two new compounds of gold and thiourea: one which he obtained from gold in a solution of thiourea with sulfuric acid and hydrogen peroxide, which he analy ...
... The structures of Ag(tu)2Cl and Au(tu)2Br have also been determined and shown to be similarly complex compounds.13,14 Moir succeeded in isolating two new compounds of gold and thiourea: one which he obtained from gold in a solution of thiourea with sulfuric acid and hydrogen peroxide, which he analy ...
precipitate - UniMAP Portal
... There are two main types of gravimetric analyses A) Precipitation analyte must first be converted to a solid (precipitate) by precipitation with an appropriate reagent. The precipitates from solution is filtered, washed, purified (if necessary) and weighed. B) Volatilization In this method the anal ...
... There are two main types of gravimetric analyses A) Precipitation analyte must first be converted to a solid (precipitate) by precipitation with an appropriate reagent. The precipitates from solution is filtered, washed, purified (if necessary) and weighed. B) Volatilization In this method the anal ...
H2 Chemistry Syllabus (9729)
... bonding. The Valence Shell Electron Pair Repulsion (VSEPR) model is used to visualise the threedimensional structure of molecules, which determines the type of interactions possible and also helps to explain the physical and chemical properties. Knowledge of structure and bonding is also important t ...
... bonding. The Valence Shell Electron Pair Repulsion (VSEPR) model is used to visualise the threedimensional structure of molecules, which determines the type of interactions possible and also helps to explain the physical and chemical properties. Knowledge of structure and bonding is also important t ...
Review of Thermodynamics
... This set of archetypal interactions can be likened to a set of ingredients that when combined create a menu that we observe as molecular recognition properties of molecules. Bringing two or more molecules together results in preferences for particular orientations that can lead to particular reactiv ...
... This set of archetypal interactions can be likened to a set of ingredients that when combined create a menu that we observe as molecular recognition properties of molecules. Bringing two or more molecules together results in preferences for particular orientations that can lead to particular reactiv ...
CHEMISTRY 101 Name Mock Final Exam Spring 2014 Signature Dr
... Consider 2 reactants, A and B. The molar mass of A is greater than the molar mass of B. You add equal masses of A and B together and let them react. Which of the following statements must be true? a) Reactant A must be limiting. b) Reactant B must be limiting. c) If the coefficient for B is greater ...
... Consider 2 reactants, A and B. The molar mass of A is greater than the molar mass of B. You add equal masses of A and B together and let them react. Which of the following statements must be true? a) Reactant A must be limiting. b) Reactant B must be limiting. c) If the coefficient for B is greater ...
CHEMISTRY SEC 06 SYLLABUS
... utilise chemical facts to illustrate a given chemical principle, concept, theory, model or pattern; apply chemical principles and patterns to make generalisations and predictions; organize, manipulate and interpret data in the form of symbols, tables, diagrams, graphs or written statements and trans ...
... utilise chemical facts to illustrate a given chemical principle, concept, theory, model or pattern; apply chemical principles and patterns to make generalisations and predictions; organize, manipulate and interpret data in the form of symbols, tables, diagrams, graphs or written statements and trans ...
Inorganometallic Chemistry
... of the main group (p-block) elements. Usually organometallic compounds are comprised not only of compounds of typical metals, but also of metalloids such as boron, silicon, arsenic, selenium, etc. In compounds of metals as well as in those of metalloids, the bond is generally polarized as follows: M ...
... of the main group (p-block) elements. Usually organometallic compounds are comprised not only of compounds of typical metals, but also of metalloids such as boron, silicon, arsenic, selenium, etc. In compounds of metals as well as in those of metalloids, the bond is generally polarized as follows: M ...
Packet 4
... When diluting a concentrated acid it is often found that combining water and the acid is a very exothermic process, i.e. one that releases energy. In some cases this energy can be very significant and may even cause the water present to turn into the gaseous state (steam). As the steam leaves the sy ...
... When diluting a concentrated acid it is often found that combining water and the acid is a very exothermic process, i.e. one that releases energy. In some cases this energy can be very significant and may even cause the water present to turn into the gaseous state (steam). As the steam leaves the sy ...
The Role of Medicinal Chemistry in Canadian Pharmacy
... Medicinal Chemistry in Pharmacy Curriculum: Conclusion Pharmaceutical/Medicinal Chemistry knowledge is essential to enrich student learning and professional outcomes Educators, stake holders and policy makers should work together to address the declining role of Pharmaceutical/Medicinal Chemistr ...
... Medicinal Chemistry in Pharmacy Curriculum: Conclusion Pharmaceutical/Medicinal Chemistry knowledge is essential to enrich student learning and professional outcomes Educators, stake holders and policy makers should work together to address the declining role of Pharmaceutical/Medicinal Chemistr ...
Reduction and Emergence in Chemistry - Philsci
... the classical theory could not, namely the power to predict how two elements might react together. Or is McLaughlin suggesting that using quantum mechanics we can predict the properties of an element from a knowledge of the number of fundamental particles that its atoms possess? Unfortunately, as an ...
... the classical theory could not, namely the power to predict how two elements might react together. Or is McLaughlin suggesting that using quantum mechanics we can predict the properties of an element from a knowledge of the number of fundamental particles that its atoms possess? Unfortunately, as an ...
Reduction and Emergence in Chemistry
... the classical theory could not, namely the power to predict how two elements might react together. Or is McLaughlin suggesting that using quantum mechanics we can predict the properties of an element from a knowledge of the number of fundamental particles that its atoms possess? Unfortunately, as an ...
... the classical theory could not, namely the power to predict how two elements might react together. Or is McLaughlin suggesting that using quantum mechanics we can predict the properties of an element from a knowledge of the number of fundamental particles that its atoms possess? Unfortunately, as an ...
Wilhelm Ostwald, the Father of Physical Chemistry
... Ostwald had the greatest passion for his work on catalysis for which he was awarded the Nobel Prize in 1909 [7]. During his Nobel Prize acceptance speech, he mentioned, “In my innermost being, I used to, and still do consider this part of my work the one in which the personal quality of my method of ...
... Ostwald had the greatest passion for his work on catalysis for which he was awarded the Nobel Prize in 1909 [7]. During his Nobel Prize acceptance speech, he mentioned, “In my innermost being, I used to, and still do consider this part of my work the one in which the personal quality of my method of ...
Chemistry 201 - Department of Chemistry | Oregon State University
... Instructions: You should have with you several number two pencils, an eraser, your 3" x 5" note card, a calculator, and your University ID Card. If you have notes with you, place them in a sealed backpack and place the backpack OUT OF SIGHT or place the notes directly on the table at the front of th ...
... Instructions: You should have with you several number two pencils, an eraser, your 3" x 5" note card, a calculator, and your University ID Card. If you have notes with you, place them in a sealed backpack and place the backpack OUT OF SIGHT or place the notes directly on the table at the front of th ...
physical setting chemistry
... 27 The acidity or alkalinity of an unknown aqueous solution is indicated by its (1) pH value (2) electronegativity value (3) percent by mass concentration (4) percent by volume concentration ...
... 27 The acidity or alkalinity of an unknown aqueous solution is indicated by its (1) pH value (2) electronegativity value (3) percent by mass concentration (4) percent by volume concentration ...
Feasibility Study of using FAIMS to Detect Carbonyl Sulfide in Propane
... Sample preparation and introduction FAIMS can be used to detect volatiles in aqueous, solid and gaseous matrices and can consequently be used for a wide variety of applications. The user requirements and sample matrix for each application define the sample preparation and introduction steps required ...
... Sample preparation and introduction FAIMS can be used to detect volatiles in aqueous, solid and gaseous matrices and can consequently be used for a wide variety of applications. The user requirements and sample matrix for each application define the sample preparation and introduction steps required ...
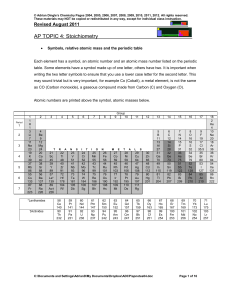
Topic #4 Notes
... each element are present in the compound rather than just the simplest whole number ratio. It is a simple multiple of the empirical formula. Hence, in the example of an empirical formula of CH2O, the molecular formula could be C2H4O2 or C3H6O3 etc. To find the molecular formula it is necessary to kn ...
... each element are present in the compound rather than just the simplest whole number ratio. It is a simple multiple of the empirical formula. Hence, in the example of an empirical formula of CH2O, the molecular formula could be C2H4O2 or C3H6O3 etc. To find the molecular formula it is necessary to kn ...
Test - Regents
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
DEPARTMENT OF CHEMISTRY Requirements For Chemistry Major
... Courses For Chemistry Minor Students intending to minor in Chemistry must currently fulfill the following: a) They must take all Year I core Chemistry Courses. b) They must earn at least 6 Credit Units from any Year II courses. c) They must earn at least 4 Credit Units from any Year III courses ...
... Courses For Chemistry Minor Students intending to minor in Chemistry must currently fulfill the following: a) They must take all Year I core Chemistry Courses. b) They must earn at least 6 Credit Units from any Year II courses. c) They must earn at least 4 Credit Units from any Year III courses ...
Document
... Chemistry deals with the matter and the change occurring in it chemists are particularly interested in these changes where one or more substances are changed in to quite different substances. They had found that these chemical changes are governed by some empirical law known as law of chemical combi ...
... Chemistry deals with the matter and the change occurring in it chemists are particularly interested in these changes where one or more substances are changed in to quite different substances. They had found that these chemical changes are governed by some empirical law known as law of chemical combi ...
Encoded Digital Periodic Table
... because it may be very useful for developing new methods of research in chemistry. Digital image of the chemistry is an image in which biochemistry is converted to mathematics. Actually, we convert it to numbers. As soon as we convert to numbers we’ll get digital image of the chemistry. In that digi ...
... because it may be very useful for developing new methods of research in chemistry. Digital image of the chemistry is an image in which biochemistry is converted to mathematics. Actually, we convert it to numbers. As soon as we convert to numbers we’ll get digital image of the chemistry. In that digi ...
Slide 1
... Gibbs Free Energy An identical procedure can be used to calculate the DGorxn given the DGof for the different chemical COMPOUNDS involved in the reaction: ...
... Gibbs Free Energy An identical procedure can be used to calculate the DGorxn given the DGof for the different chemical COMPOUNDS involved in the reaction: ...
Analytical chemistry

Analytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample, and quantitative analysis determines the amount of certain components in the substance. The separation of components is often performed prior to analysis.Analytical methods can be separated into classical and instrumental. Classical methods (also known as wet chemistry methods) use separations such as precipitation, extraction, and distillation and qualitative analysis by color, odor, or melting point. Classical quantitative analysis is achieved by measurement of weight or volume. Instrumental methods use an apparatus to measure physical quantities of the analyte such as light absorption, fluorescence, or conductivity. The separation of materials is accomplished using chromatography, electrophoresis or field flow fractionation methods.Analytical chemistry is also focused on improvements in experimental design, chemometrics, and the creation of new measurement tools to provide better chemical information. Analytical chemistry has applications in forensics, bioanalysis, clinical analysis, environmental analysis, and materials analysis.