Atomic Structure
... 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. 3. Atoms of different elements can combine with another in simple whole number ratios to form compounds. 4. Chemical reactions occur when atoms are separated, joined, or rearranged ...
... 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. 3. Atoms of different elements can combine with another in simple whole number ratios to form compounds. 4. Chemical reactions occur when atoms are separated, joined, or rearranged ...
4.1Atoms and Isotopes
... abundant), C-13 (used in medical imagingMRI), and C-14 (used for dating fossils) Tin (Sn) has the most isotopes of any element at 10 Many isotopes are radioactive (unstable nucleus that will eventually break apart and release energy in sometimes harmful forms – eg. Gamma rays) Any isotope with an at ...
... abundant), C-13 (used in medical imagingMRI), and C-14 (used for dating fossils) Tin (Sn) has the most isotopes of any element at 10 Many isotopes are radioactive (unstable nucleus that will eventually break apart and release energy in sometimes harmful forms – eg. Gamma rays) Any isotope with an at ...
Summer - Honors Chemistry
... anions, and the name of that ion is given by adding “-ide” to the root of the element name (e.g. O-2 is oxide). If an atom loses electrons, it becomes positive and is called a cation. Metals form cations, and the name of the ion is the same as the metal. If more than one charge for a metal is possib ...
... anions, and the name of that ion is given by adding “-ide” to the root of the element name (e.g. O-2 is oxide). If an atom loses electrons, it becomes positive and is called a cation. Metals form cations, and the name of the ion is the same as the metal. If more than one charge for a metal is possib ...
only that they did. democritus, an early greek philosopher, even had
... Being asked what animal you'd like to be is a trick question; you're already an animal. Doug Coupland ...
... Being asked what animal you'd like to be is a trick question; you're already an animal. Doug Coupland ...
Earth`s Chemistry PowerPoint
... • The basic building blocks of matter are atoms. Atoms consist of protons, neutrons, and electrons. • Protons have a positive electrical charge, electrons have a negative electrical charge, and neutrons are electrically neutral. Protons and neutrons make up the nucleus of an atom; electrons surround ...
... • The basic building blocks of matter are atoms. Atoms consist of protons, neutrons, and electrons. • Protons have a positive electrical charge, electrons have a negative electrical charge, and neutrons are electrically neutral. Protons and neutrons make up the nucleus of an atom; electrons surround ...
Chapter 3: Atoms
... 2. Neutrons - neutrally charged particles. 3. Electrons - negatively charged particles. B. Discovery of the Electron 1. The electron was the first subatomic particle to be discovered. a. When current is passed through a cathode-ray tube the surface of the tube opposite the cathode glowed. This glow ...
... 2. Neutrons - neutrally charged particles. 3. Electrons - negatively charged particles. B. Discovery of the Electron 1. The electron was the first subatomic particle to be discovered. a. When current is passed through a cathode-ray tube the surface of the tube opposite the cathode glowed. This glow ...
Atomic theory
... • Atomic Mass number is equal to the total number of particles in the nucleus, which is the total number of protons and neutrons in the nucleus of the atom. number of protons and neutrons (mass number) = 11 minus the number of protons (atomic number) = - 5 number of neutrons = 6 ...
... • Atomic Mass number is equal to the total number of particles in the nucleus, which is the total number of protons and neutrons in the nucleus of the atom. number of protons and neutrons (mass number) = 11 minus the number of protons (atomic number) = - 5 number of neutrons = 6 ...
HCC4 Chapter 4 Objectives and Notes
... are only distantly related to Klingons (See Star Trek - the original series). ...
... are only distantly related to Klingons (See Star Trek - the original series). ...
Radioactivity2015
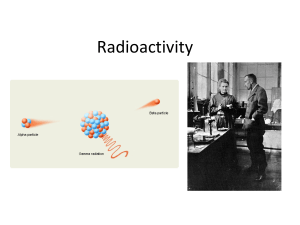
... • The helium nucleus is known as an alpha particle. This process is known as emission. In this case, an atom of uranium changes into an atom of thorium. • The general process of atoms changing into different elements is known as transmutation. • These alpha particles are less dangerous than other ...
... • The helium nucleus is known as an alpha particle. This process is known as emission. In this case, an atom of uranium changes into an atom of thorium. • The general process of atoms changing into different elements is known as transmutation. • These alpha particles are less dangerous than other ...
200 Ways to Pass the Chemistry - Home 15-16
... 85. As the pressure exerted on a gas increases, the volume decreases proportionally. 25 L of a gas is held at 1.2 atm pressure. Find the new volume if pressure drops to 0.80 atm at constant temperature. 86. As the pressure on a gas increases, temperature increases. A sample of gas exerts a pressure ...
... 85. As the pressure exerted on a gas increases, the volume decreases proportionally. 25 L of a gas is held at 1.2 atm pressure. Find the new volume if pressure drops to 0.80 atm at constant temperature. 86. As the pressure on a gas increases, temperature increases. A sample of gas exerts a pressure ...
Test 4 Review
... Covalent Bonds. Covalent bonds are bonds formed by sharing electrons. The electrons of one atom are attracted to the protons of another, but neither atom pulls strongly enough to remove an electron from the other. Covalent bonds form when the electronegativity difference between the elements is less ...
... Covalent Bonds. Covalent bonds are bonds formed by sharing electrons. The electrons of one atom are attracted to the protons of another, but neither atom pulls strongly enough to remove an electron from the other. Covalent bonds form when the electronegativity difference between the elements is less ...
atom - SCHOOLinSITES
... subatomic particle located in the nucleus and having a mass slightly less than that of a neutron. Electron differ by the number of protons in their atoms. molecules Units of two or more atoms held together by chemical bonds. The combination of atoms by chemical bonds with the component atoms in defi ...
... subatomic particle located in the nucleus and having a mass slightly less than that of a neutron. Electron differ by the number of protons in their atoms. molecules Units of two or more atoms held together by chemical bonds. The combination of atoms by chemical bonds with the component atoms in defi ...
File
... The Trend of Ionization Energy… Ionization energy increases as you go left to right on the periodic table. As you go let to right, the radius of the atom is smaller because of the greater attraction between the protons and electrons. The electrons are being held more tightly and closely by the ...
... The Trend of Ionization Energy… Ionization energy increases as you go left to right on the periodic table. As you go let to right, the radius of the atom is smaller because of the greater attraction between the protons and electrons. The electrons are being held more tightly and closely by the ...
Atoms - Pleasantville High School
... that the atoms were mostly empty space, but had a dense central core. • Why: He knew that atoms had positive and negative particles, but could not decide how they were arranged. ...
... that the atoms were mostly empty space, but had a dense central core. • Why: He knew that atoms had positive and negative particles, but could not decide how they were arranged. ...
Chemistry Midterm Review 2006
... 1. Draw a Bohr Model for sodium-24. Include the correct number of protons and neutrons in nucleus and the proper number of electrons in the energy levels. 2. What is a valence electron? 3. How many valence electrons are in the highest energy level of each? a. barium b. sodium c. aluminum d. oxygen. ...
... 1. Draw a Bohr Model for sodium-24. Include the correct number of protons and neutrons in nucleus and the proper number of electrons in the energy levels. 2. What is a valence electron? 3. How many valence electrons are in the highest energy level of each? a. barium b. sodium c. aluminum d. oxygen. ...
Isotopes File - Northwest ISD Moodle
... Ex: Magnesium has 12 protons and 12 electrons in the neutral state. (How do we know this? Answer this question.) If Magnesium has 12 protons, but only 10 electrons (which is usually the case when Mg forms an ion), is Mg more negative or more positive? Answer: More positive because Mg now has 2 more ...
... Ex: Magnesium has 12 protons and 12 electrons in the neutral state. (How do we know this? Answer this question.) If Magnesium has 12 protons, but only 10 electrons (which is usually the case when Mg forms an ion), is Mg more negative or more positive? Answer: More positive because Mg now has 2 more ...
KEY - Mrs. Bonanno`s Chemistry Resources
... “Isotopes” refer to atoms of the same element that have different mass values. This is a result of having a different number of neutrons in the nucleus. ...
... “Isotopes” refer to atoms of the same element that have different mass values. This is a result of having a different number of neutrons in the nucleus. ...
electrons - River Dell Regional School District
... however, did bounce away from the gold sheet as if they had hit ...
... however, did bounce away from the gold sheet as if they had hit ...
Mileposts on the road to the atom
... The decline of Greek civilization saw a concomitant decline of intellectual activity in Europe The major “scientific” activity was alchemy, largely the pursuit of the transformation of matter into gold Uncritical acceptance of Greek thinking about matter lingered until the Age of Enlightenment ...
... The decline of Greek civilization saw a concomitant decline of intellectual activity in Europe The major “scientific” activity was alchemy, largely the pursuit of the transformation of matter into gold Uncritical acceptance of Greek thinking about matter lingered until the Age of Enlightenment ...
File
... Mass Number Mass number: (symbol: A) total number of protons and neutrons in the nucleus (not listed on the periodic table, since it varies). NOTE this number is a whole number. Atomic Mass is different. Atoms of the same element have the same atomic number, but may have different mass numbers. ...
... Mass Number Mass number: (symbol: A) total number of protons and neutrons in the nucleus (not listed on the periodic table, since it varies). NOTE this number is a whole number. Atomic Mass is different. Atoms of the same element have the same atomic number, but may have different mass numbers. ...
Chapter 3 Notes 2015
... discoveries of Democritus, Dalton, Thomson, and Rutherford. I can describe Thomson’s and Rutherford’s model of the atom and the know the subatomic particles involved. ...
... discoveries of Democritus, Dalton, Thomson, and Rutherford. I can describe Thomson’s and Rutherford’s model of the atom and the know the subatomic particles involved. ...
AP Chemistry Chapter 2 - Anderson School District One
... to which within the molecule • Perspective drawing: gives some sense of three dimensional shape of the molecule. Solid lines represent bonds in the plane of the paper, the solid wedge is a bond that extends out of the paper and the dotted line is a bond behind the paper. • Ball and stick model: atom ...
... to which within the molecule • Perspective drawing: gives some sense of three dimensional shape of the molecule. Solid lines represent bonds in the plane of the paper, the solid wedge is a bond that extends out of the paper and the dotted line is a bond behind the paper. • Ball and stick model: atom ...