Key - Seattle Central College
... – Actinide series: Th-Lr, also called transuranium elements, generally all man-made and exist for only very short periods of time before decaying to other elements Periodic Law: ...
... – Actinide series: Th-Lr, also called transuranium elements, generally all man-made and exist for only very short periods of time before decaying to other elements Periodic Law: ...
"stuff" that takes up space- is made of tiny particles called atoms
... * Atoms are tiny. About one million (1,000,000) atoms would fit across the thickness of a human hair. ...
... * Atoms are tiny. About one million (1,000,000) atoms would fit across the thickness of a human hair. ...
المرحلة الثانية / فيزياء المحاضرة الثامنة E
... uncuttable, or indivisible, something that cannot be divided further. The concept of an atom as an indivisible component of matter was first proposed by early Indian and Greek philosophers. In the 17th and 18th centuries, chemists provided a physical basis for this idea by showing that certain subst ...
... uncuttable, or indivisible, something that cannot be divided further. The concept of an atom as an indivisible component of matter was first proposed by early Indian and Greek philosophers. In the 17th and 18th centuries, chemists provided a physical basis for this idea by showing that certain subst ...
atoms
... and neutrons. Some nuclei are unstable because they have too many or too few neutrons. This is especially true for heavier elements such as uranium and plutonium. • The release of nuclear particles and energy is called radioactive decay. • In these nuclei, repulsion builds up. The nucleus must relea ...
... and neutrons. Some nuclei are unstable because they have too many or too few neutrons. This is especially true for heavier elements such as uranium and plutonium. • The release of nuclear particles and energy is called radioactive decay. • In these nuclei, repulsion builds up. The nucleus must relea ...
elements and isotopes - vocabulary
... An isotope whose atoms undergo a detectable radioactive decay. Radioactive isotopes can be naturally occurring or synthetic. radioactive decay Transformation of an unstable atom that produces a more stable atom (or atoms) as well as radiation (energy and energetic particles). synthetic element (isot ...
... An isotope whose atoms undergo a detectable radioactive decay. Radioactive isotopes can be naturally occurring or synthetic. radioactive decay Transformation of an unstable atom that produces a more stable atom (or atoms) as well as radiation (energy and energetic particles). synthetic element (isot ...
Name: Date: Chemistry 1 – Midterm Review Sheet Unit 1 – Scientific
... 3. The energy levels of the hydrogen atom (and all atoms) are ______________, meaning that only certain discrete energy levels are allowed. a. varied b. quantized c. ramp-like d. continuous e. two of these 4. The form of EMR that has less energy than microwaves is a. microwaves b. radio waves c. ga ...
... 3. The energy levels of the hydrogen atom (and all atoms) are ______________, meaning that only certain discrete energy levels are allowed. a. varied b. quantized c. ramp-like d. continuous e. two of these 4. The form of EMR that has less energy than microwaves is a. microwaves b. radio waves c. ga ...
Chemistry 1st Grading Period Notes 090211 Pointers Topics Identify
... states that energy levels must be filled from the lowest to highest and you may not move on the next level unless the previous level is full 5 electrons- 1s2 2s2 2p1 Calcium 20- 1s2 2s2 2p6 3s2 3p6 4s2 4s2 before d because it is shielded by the attractive force of the proton Noble Gases ...
... states that energy levels must be filled from the lowest to highest and you may not move on the next level unless the previous level is full 5 electrons- 1s2 2s2 2p1 Calcium 20- 1s2 2s2 2p6 3s2 3p6 4s2 4s2 before d because it is shielded by the attractive force of the proton Noble Gases ...
Model Timeline Project Atomic Model Scientists Timeline
... 4. Your timeline should include AT LEAST the following scientists: Democritus, Aristotle, John Dalton, .James Chadwick, Ernest Rutherford, J.J. Thomson, Dmitri Mendeleev, the French chemist Lavoisier, Michael Faraday, Robert A. Millikan, Erwin Schrödinger and Max Planck 5. It must be neat and legibl ...
... 4. Your timeline should include AT LEAST the following scientists: Democritus, Aristotle, John Dalton, .James Chadwick, Ernest Rutherford, J.J. Thomson, Dmitri Mendeleev, the French chemist Lavoisier, Michael Faraday, Robert A. Millikan, Erwin Schrödinger and Max Planck 5. It must be neat and legibl ...
Atoms, Molecules, and Ions
... can be referred to collectively by their group number such as 2A or special names that the groups have been give. Group 1A elements are called alkali metals, group 2A elements are called alkaline earth metals, group 7A elements are known as halogens, and group 8A elements are called noble gasses. We ...
... can be referred to collectively by their group number such as 2A or special names that the groups have been give. Group 1A elements are called alkali metals, group 2A elements are called alkaline earth metals, group 7A elements are known as halogens, and group 8A elements are called noble gasses. We ...
Chapter 4 Notes - Atomic Theory
... Ions: Atoms that gain and lose electrons to become stable (full valence shells). 1. Cations: metals that lose electrons & form positive ions (Na+) Multivalent: Some metals can have more than one charge (Fe2+ or Fe3+). 2. Anions: Non-metals gain electrons & form negative ions (O-2) ...
... Ions: Atoms that gain and lose electrons to become stable (full valence shells). 1. Cations: metals that lose electrons & form positive ions (Na+) Multivalent: Some metals can have more than one charge (Fe2+ or Fe3+). 2. Anions: Non-metals gain electrons & form negative ions (O-2) ...
Atomic Theory
... chemist, studied the results of experiments by Lavoisier and many other scientists. • Dalton proposed his atomic theory of matter in 1803. ...
... chemist, studied the results of experiments by Lavoisier and many other scientists. • Dalton proposed his atomic theory of matter in 1803. ...
Chapter 4 and 5 Powerpoint - School District of La Crosse
... an electron can have in the atom. Level 1= 2 e: level 2=8e: level 3= 18e: level 4=32: level 5=50 1. electrons gain energy to move to a higher level and give up energy when they drop back into a lower level( ground state) 1. No longer assumed the electron moved in definite orbitals( Heisenberg uncert ...
... an electron can have in the atom. Level 1= 2 e: level 2=8e: level 3= 18e: level 4=32: level 5=50 1. electrons gain energy to move to a higher level and give up energy when they drop back into a lower level( ground state) 1. No longer assumed the electron moved in definite orbitals( Heisenberg uncert ...
Intro to Chemistry
... The number of protons, electrons, and neutrons in an element predicts their behavior under a variety of conditions inside and outside the body. ...
... The number of protons, electrons, and neutrons in an element predicts their behavior under a variety of conditions inside and outside the body. ...
Worksheet 4.1 File
... a. All elements are composed of tiny, indivisible particles called atoms. b. An element is composed of several types of atoms. c. Atoms of different elements can physically mix together, or can chemically combine in simple, whole-number ratios to form compounds. d. Chemical reactions occur when atom ...
... a. All elements are composed of tiny, indivisible particles called atoms. b. An element is composed of several types of atoms. c. Atoms of different elements can physically mix together, or can chemically combine in simple, whole-number ratios to form compounds. d. Chemical reactions occur when atom ...
atomic number
... identical. Atoms of any one element are different from those of any other element. ...
... identical. Atoms of any one element are different from those of any other element. ...
Abstract
... because they have the same number of electrons. Therefore, isotope ratios such as 18O/16O change little during chemical reactions. However, in earth and planetary science, the subtle changes of isotope ratios help a lot in elucidating the origin and formation of planetary materials. For expression o ...
... because they have the same number of electrons. Therefore, isotope ratios such as 18O/16O change little during chemical reactions. However, in earth and planetary science, the subtle changes of isotope ratios help a lot in elucidating the origin and formation of planetary materials. For expression o ...
Name
... a. All elements are composed of tiny, indivisible particles called atoms. b. An element is composed of several types of atoms. c. Atoms of different elements can physically mix together, or can chemically combine in simple, whole-number ratios to form compounds. d. Chemical reactions occur when atom ...
... a. All elements are composed of tiny, indivisible particles called atoms. b. An element is composed of several types of atoms. c. Atoms of different elements can physically mix together, or can chemically combine in simple, whole-number ratios to form compounds. d. Chemical reactions occur when atom ...
Name
... 4. Atoms are the _____________________________________________ C. What’s in an atom? 1. Atoms are made of _________________________________________ a. At the center of each atom is a __________________________ ______________________________________________________ b. The nucleus is made of _______ ( ...
... 4. Atoms are the _____________________________________________ C. What’s in an atom? 1. Atoms are made of _________________________________________ a. At the center of each atom is a __________________________ ______________________________________________________ b. The nucleus is made of _______ ( ...
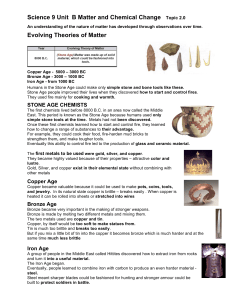
An understanding of the nature of matter has developed
... Once these first chemists learned how to start and control fire, they learned how to change a range of substances to their advantage. For example, they could cook their food, fire-harden mud bricks to strengthen them, and make tougher tools. Eventually this ability to control fire led to the product ...
... Once these first chemists learned how to start and control fire, they learned how to change a range of substances to their advantage. For example, they could cook their food, fire-harden mud bricks to strengthen them, and make tougher tools. Eventually this ability to control fire led to the product ...
Chemical Reactions
... whose solutes do not settle out Suspensions – heterogeneous mixtures with visible solutes that tend to settle out ...
... whose solutes do not settle out Suspensions – heterogeneous mixtures with visible solutes that tend to settle out ...
Entry Task
... – Semiconductors-special materials that conduct electricity under some conditions and not under others ...
... – Semiconductors-special materials that conduct electricity under some conditions and not under others ...
1 - VCE Chemistry
... 4. A group in the periodic table is identified as: A) Elements with the same electronegativity B) A column of the periodic table C) A row of the periodic table D) Elements with different numbers of valence electrons E) Elements with identical electronic configurations 5. Bromine and iodine are in th ...
... 4. A group in the periodic table is identified as: A) Elements with the same electronegativity B) A column of the periodic table C) A row of the periodic table D) Elements with different numbers of valence electrons E) Elements with identical electronic configurations 5. Bromine and iodine are in th ...
Greek philosophers (300 BC)
... Atoms are arranged in energy levels (e.l.’s), at different distances from nucleus Close to nucleus = low energy Far from nucleus = high energy e-s in highest occupied level are “valence e-s” Only so many e-’s can fit in energy levels e-s fill lower e.l.’s before being located in higher e. ...
... Atoms are arranged in energy levels (e.l.’s), at different distances from nucleus Close to nucleus = low energy Far from nucleus = high energy e-s in highest occupied level are “valence e-s” Only so many e-’s can fit in energy levels e-s fill lower e.l.’s before being located in higher e. ...