Solutions
... ‣ The quick story is molecules have a negative end and a positive end. ‣ The negative end of one molecule sticks to the positive end of another. ‣ We’ll discuss the rest in Chapter 11. ‣ Ionic Solids are held together by one type of intermolecular force. ‣ It’s a simpler story. ‣ The cations stick t ...
... ‣ The quick story is molecules have a negative end and a positive end. ‣ The negative end of one molecule sticks to the positive end of another. ‣ We’ll discuss the rest in Chapter 11. ‣ Ionic Solids are held together by one type of intermolecular force. ‣ It’s a simpler story. ‣ The cations stick t ...
Solubility Solubility is defined as the amount of solute that will
... final time - initial time min This will give us a average rate because we will find that often the rate will change as the reaction proceeds. Also you might observe that the negative sign in front of the equation makes the value positive, as required by the definitition of rate. The rate is the expe ...
... final time - initial time min This will give us a average rate because we will find that often the rate will change as the reaction proceeds. Also you might observe that the negative sign in front of the equation makes the value positive, as required by the definitition of rate. The rate is the expe ...
ksp - lozon.ca
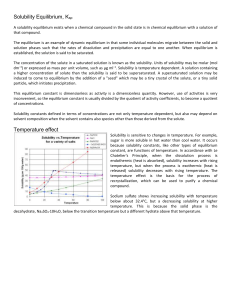
... The concentration of the solute in a saturated solution is known as the solubility. Units of solubility may be molar (mol dm−3) or expressed as mass per unit volume, such as μg ml−1. Solubility is temperature dependent. A solution containing a higher concentration of solute than the solubility is sa ...
... The concentration of the solute in a saturated solution is known as the solubility. Units of solubility may be molar (mol dm−3) or expressed as mass per unit volume, such as μg ml−1. Solubility is temperature dependent. A solution containing a higher concentration of solute than the solubility is sa ...
Lanthanide complexes
... center. This difference has been attributed to the lack of core-valence correlation with a large-core ECP and not to the participation of the 4f electrons. The lack of participation of the 4f electrons in bonding is also evidenced by the calculated 4f orbital occupancy. The total number of 4f electr ...
... center. This difference has been attributed to the lack of core-valence correlation with a large-core ECP and not to the participation of the 4f electrons. The lack of participation of the 4f electrons in bonding is also evidenced by the calculated 4f orbital occupancy. The total number of 4f electr ...
Chapter 17, 18 Lecture
... Ethers. A family of molecules with a general formula : R-O-R (symmetrical ethers) or R-O-R' (unsymmetrical ethers). Due to the presence of lone electron pairs on an O atom, this molecules are modestly polar and can serve as acceptors of H-bond. One use of ethers as solvents is to stabilize the metal ...
... Ethers. A family of molecules with a general formula : R-O-R (symmetrical ethers) or R-O-R' (unsymmetrical ethers). Due to the presence of lone electron pairs on an O atom, this molecules are modestly polar and can serve as acceptors of H-bond. One use of ethers as solvents is to stabilize the metal ...
Exploring Oxidovanadium(IV) - ACS Publications
... lower than 1.5 Å, whereas two different poses were found for 2, one of which is highly similar to the binding mode predicted for 1 and was therefore accounted for in results analysis (for the other binding conformation of 2, see Supporting Information). Molecules 1−3 bind within the catalytic pocket ...
... lower than 1.5 Å, whereas two different poses were found for 2, one of which is highly similar to the binding mode predicted for 1 and was therefore accounted for in results analysis (for the other binding conformation of 2, see Supporting Information). Molecules 1−3 bind within the catalytic pocket ...
Nanostructured Transition Metal Chalcogenides as - KIT
... The electrochemical behaviour of the new ternary material ‘Li2Cu4S3’ was investigated in Swagelock Cells under conditions like discussed before at first in a region between 1.5V and 3V. The voltage profile of two cycles is presented in Fig. 9. This shows that the discharge reaction consists of two s ...
... The electrochemical behaviour of the new ternary material ‘Li2Cu4S3’ was investigated in Swagelock Cells under conditions like discussed before at first in a region between 1.5V and 3V. The voltage profile of two cycles is presented in Fig. 9. This shows that the discharge reaction consists of two s ...
Stable gold(III) catalysts by oxidative addition of a carbon
... Full conversion to [IPrAu(III)(Me4-biphenyl)(H2O)][SbF6] (compound 7) was observed in 5 min at room temperature. When replacing AgSbF6 with AgOTf, the oxidative addition was complete after 6 h, giving [IPrAu (III)(Me4-biphenyl)OTf] (compound 12). The longer reaction time required in the presence of ...
... Full conversion to [IPrAu(III)(Me4-biphenyl)(H2O)][SbF6] (compound 7) was observed in 5 min at room temperature. When replacing AgSbF6 with AgOTf, the oxidative addition was complete after 6 h, giving [IPrAu (III)(Me4-biphenyl)OTf] (compound 12). The longer reaction time required in the presence of ...
Synthesis of Chiral C2-symmetric Palladium and Rhodium SCS
... triflate (Me3SnOTf) to afford organotin compound 11 in 47% yield (Scheme 1).7 Finally, with the precursors of pincers, 10 and 11, in hand, we tried to synthesize the corresponding C2-symmetric SCS pincers of various metals (Scheme 2). The organopalladium(II) complex 12 was prepared directly from the ...
... triflate (Me3SnOTf) to afford organotin compound 11 in 47% yield (Scheme 1).7 Finally, with the precursors of pincers, 10 and 11, in hand, we tried to synthesize the corresponding C2-symmetric SCS pincers of various metals (Scheme 2). The organopalladium(II) complex 12 was prepared directly from the ...
chapter 16
... Relative energies of reactants and products (nature goes to minimum energy) Degree of organization of reactants and products (nature goes to maximum disorder) The significance of K: K> 1 means that the reaction favors the products at equilibrium K < 1 means that the reaction favors the reactan ...
... Relative energies of reactants and products (nature goes to minimum energy) Degree of organization of reactants and products (nature goes to maximum disorder) The significance of K: K> 1 means that the reaction favors the products at equilibrium K < 1 means that the reaction favors the reactan ...
Complexes of the Group 5 Elements
... Paddlewheel compounds having two dimetal units, each with a square planar configuration, and each group parallel to the other are relatively new in group 5. With other transition metal atoms, this type of atom arrangement is commonly found for M2n+ units where n = 4, 5 or 6. This means that the oxida ...
... Paddlewheel compounds having two dimetal units, each with a square planar configuration, and each group parallel to the other are relatively new in group 5. With other transition metal atoms, this type of atom arrangement is commonly found for M2n+ units where n = 4, 5 or 6. This means that the oxida ...
In Class Problems and Notes AP Chemistry General Equilibrium
... (c) Write the equilibrium expression for the equilibrium constant, KP, and calculate its value for the reaction under the conditions in (a). (d) If 110. grams of solid NaHCO3 had been placed in the 5.00-liter container and heated to 160ºC, what would the total pressure have been at equilibrium? Expl ...
... (c) Write the equilibrium expression for the equilibrium constant, KP, and calculate its value for the reaction under the conditions in (a). (d) If 110. grams of solid NaHCO3 had been placed in the 5.00-liter container and heated to 160ºC, what would the total pressure have been at equilibrium? Expl ...
Lab Handout
... summarises what the table is showing. When investigating, the order of the entries is arbitrary. When reporting results, they should be sorted into an order. Show all set-ups for each type of calculation: be explicit. If you have to perform the same calculation more than once you do not have to writ ...
... summarises what the table is showing. When investigating, the order of the entries is arbitrary. When reporting results, they should be sorted into an order. Show all set-ups for each type of calculation: be explicit. If you have to perform the same calculation more than once you do not have to writ ...
AP Thermodynamics ppt.
... • Choose the sample of matter that has the greater entropy in each pair and explain your choice: • a) 1 mol of solid NaCl or 1 mol of gaseous HCl at 25C • b) 2 mol of HCl(g) or 1 mol of HCl(g) at 25C • c) 1 mol of HCl(g) or 1 mol of Ar(g) at 25C • d) 1 mol of N2(s) at 24 K or 1 mol of N2 at 25C ...
... • Choose the sample of matter that has the greater entropy in each pair and explain your choice: • a) 1 mol of solid NaCl or 1 mol of gaseous HCl at 25C • b) 2 mol of HCl(g) or 1 mol of HCl(g) at 25C • c) 1 mol of HCl(g) or 1 mol of Ar(g) at 25C • d) 1 mol of N2(s) at 24 K or 1 mol of N2 at 25C ...
Reactivity of coordinated peroxide at a highly peroxygenated
... salt. This was highly soluble and stable in water. The results of chemical analyses suggested the stoichiometry of K:V:022- as 1 :1:2, and the molar conductance in water was found to be 135 R-' cm2 mol-', clearly indicating a 1:1 electrolytic nature of the product. On the basis of this information a ...
... salt. This was highly soluble and stable in water. The results of chemical analyses suggested the stoichiometry of K:V:022- as 1 :1:2, and the molar conductance in water was found to be 135 R-' cm2 mol-', clearly indicating a 1:1 electrolytic nature of the product. On the basis of this information a ...
An Efficient Oxidation of Benzoins to Benzils by Manganese (II
... Schiff base complexes of transition metals having O and N donor atoms have shown an exponential increase as inorganic catalysts for various organic transformations. Distinct advantages of such ligands include their low cost, facile syntheses, and convenient incorporation of inexpensive, chiral 1,2-di ...
... Schiff base complexes of transition metals having O and N donor atoms have shown an exponential increase as inorganic catalysts for various organic transformations. Distinct advantages of such ligands include their low cost, facile syntheses, and convenient incorporation of inexpensive, chiral 1,2-di ...
Homework Solutions Week 6
... When silver sulfate starts to precipitate, 97% of the calcium has precipitated. And when calcium from 97 to 99% precipitated, silver ion goes from 0 to 41% precipitated. 9-17 a) Why do many rivers in Box 9-1. lie on the line [HCO3-] = 2[Ca2+]? According to Box 9-1, the source of calcium in the river ...
... When silver sulfate starts to precipitate, 97% of the calcium has precipitated. And when calcium from 97 to 99% precipitated, silver ion goes from 0 to 41% precipitated. 9-17 a) Why do many rivers in Box 9-1. lie on the line [HCO3-] = 2[Ca2+]? According to Box 9-1, the source of calcium in the river ...
Synthesis and crystal structure of
... ing Tl compound. However, this In-In distance is significantly shorter than the pm in {C,H,In}, and {MeC,H,In}, interchain In . - - In distances of 3986(l) In distances of 3963(l) and 394.3(l) pm in [1,19], or the intraclusteral In. {Me,C,In},, which are interpreted in terms of weak In-In interactio ...
... ing Tl compound. However, this In-In distance is significantly shorter than the pm in {C,H,In}, and {MeC,H,In}, interchain In . - - In distances of 3986(l) In distances of 3963(l) and 394.3(l) pm in [1,19], or the intraclusteral In. {Me,C,In},, which are interpreted in terms of weak In-In interactio ...
Chapter 2 Geochemical Reactions
... implied in the term geochemistry. These processes include dissolution of air-borne material and gases, weathering at the Earth’s surface, biodegradation and nutrient cycling in the soil, mineral dissolution in the subsurface, and mixing with seawater or deep crustal water. Human activity also plays ...
... implied in the term geochemistry. These processes include dissolution of air-borne material and gases, weathering at the Earth’s surface, biodegradation and nutrient cycling in the soil, mineral dissolution in the subsurface, and mixing with seawater or deep crustal water. Human activity also plays ...
Physical Chemistry Problems. ©Mike Lyons 2009
... Write down an expression for the First Law of Thermodynamics which relates the change in internal energy of a system to the work done on the system and the heat absorbed by the system. Hence derive a relationship between the change in internal energy U and the change in enthalpy H of a system. b. ...
... Write down an expression for the First Law of Thermodynamics which relates the change in internal energy of a system to the work done on the system and the heat absorbed by the system. Hence derive a relationship between the change in internal energy U and the change in enthalpy H of a system. b. ...
Olefin Metathesis by Molybdenum lmido Alkylidene Catalysts R2H
... stability of intermediates in the catalytic cycle usually change dramatically. Perhaps the most important intermediate to consider is a methylene complex. Few methylene complexes can be isolated, or even observed in solution by NMR methods, in part because they are the most reactive alkylidenes, and ...
... stability of intermediates in the catalytic cycle usually change dramatically. Perhaps the most important intermediate to consider is a methylene complex. Few methylene complexes can be isolated, or even observed in solution by NMR methods, in part because they are the most reactive alkylidenes, and ...
J. Am. Chem. SOC. 1993,115, 7685-7695
... Reaction of [(C&le~)Rh(PMe,)l with Naphthalene and Benzene. (C5Me5)Rh(PMe3)(Ph)H (1) when heated to 60 "C serves as an efficient thermal source of the coordinatively unsaturated fragment [(CsMes)Rh(PMe3)]. In the presence of excess naphthalene, complete conversion to a 1:2 mixture of the naphthyl hy ...
... Reaction of [(C&le~)Rh(PMe,)l with Naphthalene and Benzene. (C5Me5)Rh(PMe3)(Ph)H (1) when heated to 60 "C serves as an efficient thermal source of the coordinatively unsaturated fragment [(CsMes)Rh(PMe3)]. In the presence of excess naphthalene, complete conversion to a 1:2 mixture of the naphthyl hy ...