Checking the Kinetics of Acetic Acid Production by
... constant using a batch reactor. In order to determine the acetic anhydride concentration, samples from the reactor were withdrawn into tarred flasks containing 15∼20 times the quantity of saturated aniline-water required to react with the sample. Since the anhydride rapidly acetylates the aniline, p ...
... constant using a batch reactor. In order to determine the acetic anhydride concentration, samples from the reactor were withdrawn into tarred flasks containing 15∼20 times the quantity of saturated aniline-water required to react with the sample. Since the anhydride rapidly acetylates the aniline, p ...
Chapter 13 PowerPoint
... mainly product – equilibrium lies to the right A very small K means that the system at equilibrium will consist mainly of reactants – equilibrium position is far to the left The size of K and the time required to reach equilibrium are NOT directly related. Complete sample problem #5. ...
... mainly product – equilibrium lies to the right A very small K means that the system at equilibrium will consist mainly of reactants – equilibrium position is far to the left The size of K and the time required to reach equilibrium are NOT directly related. Complete sample problem #5. ...
MS PowerPoint - Catalysis Eprints database
... and the order (participating species) The general expression for the power law model for an elementary reaction AB is written as: -rA = kCAn (where n is 1 ) This expression can be applicable to complex reactions as well. How will you write the rate expression for the following reaction if it were e ...
... and the order (participating species) The general expression for the power law model for an elementary reaction AB is written as: -rA = kCAn (where n is 1 ) This expression can be applicable to complex reactions as well. How will you write the rate expression for the following reaction if it were e ...
U-Ti alloy as a promising storage material for hydrogen isotopes
... hydrogen pressure together with the concentration of H in the metal is increased, interactions between hydrogen atoms become locally important, and we start to see nucleation and growth of the hydride (β) phase. While the two phases coexist, the isotherms show a flat plateau, the length of which det ...
... hydrogen pressure together with the concentration of H in the metal is increased, interactions between hydrogen atoms become locally important, and we start to see nucleation and growth of the hydride (β) phase. While the two phases coexist, the isotherms show a flat plateau, the length of which det ...
09_Lecture
... • The equilibrium constant, K, defines the extent of a chemical reaction as a ratio of the concentration of the products to the concentration of the reactants. • If a chemical reaction at equilibrium is disturbed, the reaction can regain its equilibrium according to Le Châtelier’s principle by shift ...
... • The equilibrium constant, K, defines the extent of a chemical reaction as a ratio of the concentration of the products to the concentration of the reactants. • If a chemical reaction at equilibrium is disturbed, the reaction can regain its equilibrium according to Le Châtelier’s principle by shift ...
2.3 Atomic and Molecular Collisions
... merged electron beam device awaits first operation in 2017. Also the neutral atomic beam setup of the CSR (positions 2, 3 and 7 in Fig. 6) is completely installed and awaits first experiments. It is fed by anion beams from a separate platform. Using a photodetachment zone with a very strong (2 kW) c ...
... merged electron beam device awaits first operation in 2017. Also the neutral atomic beam setup of the CSR (positions 2, 3 and 7 in Fig. 6) is completely installed and awaits first experiments. It is fed by anion beams from a separate platform. Using a photodetachment zone with a very strong (2 kW) c ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... Analyze: Our task is to write a net ionic equation for a precipitation reaction, given the names of the reactants present in solution. Plan: We first need to write the chemical formulas of the reactants and products and to determine which product is insoluble. Then we write and balance the molecular ...
... Analyze: Our task is to write a net ionic equation for a precipitation reaction, given the names of the reactants present in solution. Plan: We first need to write the chemical formulas of the reactants and products and to determine which product is insoluble. Then we write and balance the molecular ...
PDF document
... – no interference from other active compounds present in the commercial dosage forms, if they resist the chemical reaction conditions – free from background interferences – because measurements are often made faster with kinetic methods than equilibrium methods, many very slow reactions become analy ...
... – no interference from other active compounds present in the commercial dosage forms, if they resist the chemical reaction conditions – free from background interferences – because measurements are often made faster with kinetic methods than equilibrium methods, many very slow reactions become analy ...
Slide 1
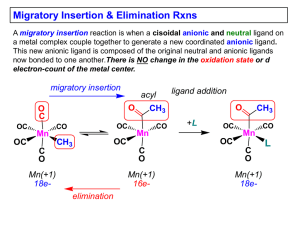
... Migratory Insertion & Elimination Rxns A migratory insertion reaction is when a cisoidal anionic and neutral ligand on a metal complex couple together to generate a new coordinated anionic ligand. This new anionic ligand is composed of the original neutral and anionic ligands now bonded to one anoth ...
... Migratory Insertion & Elimination Rxns A migratory insertion reaction is when a cisoidal anionic and neutral ligand on a metal complex couple together to generate a new coordinated anionic ligand. This new anionic ligand is composed of the original neutral and anionic ligands now bonded to one anoth ...
Full-Text PDF
... Ru-based catalysts bearing an N-heterocyclic carbene (NHC) ligand, the activation of these congeners occurs through a dissociative mechanism, with a more exothermic first phosphine dissociation step. In spite of a stronger electron-donating ability of a phosphonium ylide C-ligand with respect to a d ...
... Ru-based catalysts bearing an N-heterocyclic carbene (NHC) ligand, the activation of these congeners occurs through a dissociative mechanism, with a more exothermic first phosphine dissociation step. In spite of a stronger electron-donating ability of a phosphonium ylide C-ligand with respect to a d ...
Platinum Metals Complex Catalysts for Liquid
... a general feature typical for all the platinum metals studied. However, we do not think that the hydride, formed at an early stage of the interaction between the components, can react with a multiple bond of the substrate to start a catalytic cycle. Instead, it initiates a chain of reactions that fi ...
... a general feature typical for all the platinum metals studied. However, we do not think that the hydride, formed at an early stage of the interaction between the components, can react with a multiple bond of the substrate to start a catalytic cycle. Instead, it initiates a chain of reactions that fi ...
Manfred Scheer Coordination Chemistry of Phosphorous
... Stabilization of the [WPC2] ring - Reaction with W-CO unit (complex 6) - Reaction with 2nd unit of the intermediate (complex 4) ...
... Stabilization of the [WPC2] ring - Reaction with W-CO unit (complex 6) - Reaction with 2nd unit of the intermediate (complex 4) ...