Synthesis, Characterisation and DFT Analysis of {Ru(NO)2}8
... nitroprusside (SNP) as an example for the PLI-effect................................................................................................................12 Fig. 1.13: Some examples for mononitrosyl complexes. ................................................................................ ...
... nitroprusside (SNP) as an example for the PLI-effect................................................................................................................12 Fig. 1.13: Some examples for mononitrosyl complexes. ................................................................................ ...
The Kubas Interaction in Transition Metal Based Hydrogen Storage
... The binding sites were modelled as fragments representing the active sites in the extended structures. Evidence has been found for the hydrogen binding through the Kubas interaction and the results were benchmarked against the available experimental data. The transition metals of the binding sites a ...
... The binding sites were modelled as fragments representing the active sites in the extended structures. Evidence has been found for the hydrogen binding through the Kubas interaction and the results were benchmarked against the available experimental data. The transition metals of the binding sites a ...
Mercury(II) Removal with Modified Magnetic Chitosan Adsorbents
... conditioning, and composition of the solution), which are not systematically the same [4]. The latter is the reason why the direct comparison of experimental data is not possible. In the present study, Hg(II) was selected as target for removal with adsorption technique, which is considered to be one ...
... conditioning, and composition of the solution), which are not systematically the same [4]. The latter is the reason why the direct comparison of experimental data is not possible. In the present study, Hg(II) was selected as target for removal with adsorption technique, which is considered to be one ...
Weak Intermolecular Interactions in the Solid State
... halogen bonding is a strong, specific, and directional interaction13b that can in quite many cases overrule other intermolecular interactions, such as hydrogen bonding, π···π, cation···π and C–H···π interactions. The nature of the halogen bond, determined as the interaction of a polarized halogen at ...
... halogen bonding is a strong, specific, and directional interaction13b that can in quite many cases overrule other intermolecular interactions, such as hydrogen bonding, π···π, cation···π and C–H···π interactions. The nature of the halogen bond, determined as the interaction of a polarized halogen at ...
214. - Materials and Process Simulation Center
... GVB description, we think of the two singly occupied orbitals (s and d) as combined into sd hybrids (pointing at 90° from each other) and covalently paired with the singly occupied ligand orbital (H, CH,, or C1) to form a covalent bond pair. The presence of phosphines leads to destabilization of the ...
... GVB description, we think of the two singly occupied orbitals (s and d) as combined into sd hybrids (pointing at 90° from each other) and covalently paired with the singly occupied ligand orbital (H, CH,, or C1) to form a covalent bond pair. The presence of phosphines leads to destabilization of the ...
Atmospheric Formation_TELTEK
... 0.58 : 0.42 in CH3NH2 and CD3ND2, respectively. Since OH radicals and Cl atoms often show similar selectivity in their reactions, one may expect that also hydrogen abstraction in primary amines by OH radicals will occur from both C and N. Galano and Alvarez-Idaboy have calculated the rate constant f ...
... 0.58 : 0.42 in CH3NH2 and CD3ND2, respectively. Since OH radicals and Cl atoms often show similar selectivity in their reactions, one may expect that also hydrogen abstraction in primary amines by OH radicals will occur from both C and N. Galano and Alvarez-Idaboy have calculated the rate constant f ...
evaluation copy
... material for any class he or she teaches. No part of these activities may be used or reproduced in any other manner without prior written permission of PASCO scientific, except in the case of brief quotations used in critical articles or reviews. SPARK Science Learning System, SPARKvue, PASCO Capsto ...
... material for any class he or she teaches. No part of these activities may be used or reproduced in any other manner without prior written permission of PASCO scientific, except in the case of brief quotations used in critical articles or reviews. SPARK Science Learning System, SPARKvue, PASCO Capsto ...
Role of Pt-precursor on the performance of Pt/BaCO3/Al2O3·NOx
... with alumina, impregnated with Ba(NO3 )2 and, finally, impregnated with platinum. Four different platinum precursors were used in the preparation (one for each sample). The preparation method is described in detail in [26]. Briefly, the monoliths were coated with alumina by immersing the monolith in ...
... with alumina, impregnated with Ba(NO3 )2 and, finally, impregnated with platinum. Four different platinum precursors were used in the preparation (one for each sample). The preparation method is described in detail in [26]. Briefly, the monoliths were coated with alumina by immersing the monolith in ...
Chapter 1 Introduction to Supramolecular Chemistry
... 1.6).19 Whitesides and his coworkers then successfully capitalized on this discovery by constructing roughly a globular nanostructure from cyanuric acid 1 and melamine derivatives 2.2,20,21 Their approach to the well-defined molecular globular structure is illustrated in Figure 1.7. To overcome a la ...
... 1.6).19 Whitesides and his coworkers then successfully capitalized on this discovery by constructing roughly a globular nanostructure from cyanuric acid 1 and melamine derivatives 2.2,20,21 Their approach to the well-defined molecular globular structure is illustrated in Figure 1.7. To overcome a la ...
Adsorption of Metallic Ions onto Chitosan: Equilibrium and
... aluminium hydroxides in drinking water treatment. Metal coagulants can be used to partially remove heavy metal from wastewater (Eilbeck and Mattock, 1987), but, the use of metal coagulants is not 100% effective for removing metal cations from water at pH 7 (Bell and Saunders, 2005). The high cost of ...
... aluminium hydroxides in drinking water treatment. Metal coagulants can be used to partially remove heavy metal from wastewater (Eilbeck and Mattock, 1987), but, the use of metal coagulants is not 100% effective for removing metal cations from water at pH 7 (Bell and Saunders, 2005). The high cost of ...
... NH4F are added to the bath as the buffering agents [7]. In other studies, thiourea and lead acetate are commonly employed as stabilizers in electroless plating baths [9, 10]. Complexing agents as lactates, hydroxy-acetate, glycines, malonates and certain fluorites [11] can be added. In addition, som ...
inorganic chemistry and spectroscopy ( a systematic approach)
... INORGANIC CHEMITRYAND SPECTROSCOPY ...
... INORGANIC CHEMITRYAND SPECTROSCOPY ...
A study of complexes Mg(NH3)n and Ag(NH3)n , where n 1–8
... for sequential addition of NH3 to Mg⫹䡠 resulting from direct coordination to the metal are 38.1, 26.6, 21.2, 13.7, 12.1, and 11.3 kcal mol⫺1. The free energies for these same addition reactions are all negative, although for complexes with n ⱖ 4 the values are very small. Attempts at optimising stru ...
... for sequential addition of NH3 to Mg⫹䡠 resulting from direct coordination to the metal are 38.1, 26.6, 21.2, 13.7, 12.1, and 11.3 kcal mol⫺1. The free energies for these same addition reactions are all negative, although for complexes with n ⱖ 4 the values are very small. Attempts at optimising stru ...
Paving the Way to The Integration of Smart Nanostructures:
... decomposition, spray pyrolysis, sol-gel routes and freeze drying, precipitation or electrodeposition from solution. Despite all this intense activity the mechanism of the OER at first row transition metal oxide surfaces remains controversial. The experimental confirmation that a common OER mechanism ...
... decomposition, spray pyrolysis, sol-gel routes and freeze drying, precipitation or electrodeposition from solution. Despite all this intense activity the mechanism of the OER at first row transition metal oxide surfaces remains controversial. The experimental confirmation that a common OER mechanism ...
18 Chapter 3 Structures of Coordination Compounds Problem
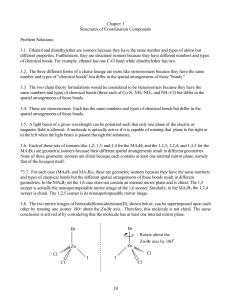
... 3.1. Ethanol and dimethylether are isomers because they have the same number and types of atoms but different properties. Furthermore, they are structural isomers because they have different numbers and types of chemical bonds. For example, ethanol has one C-O bond while dimethylether has two. 3.2. ...
... 3.1. Ethanol and dimethylether are isomers because they have the same number and types of atoms but different properties. Furthermore, they are structural isomers because they have different numbers and types of chemical bonds. For example, ethanol has one C-O bond while dimethylether has two. 3.2. ...
4134gdisk doc..4134gdisk chapter .. Page501
... by [Ru(terpy)(bpy)O]2+ type complexes have been found to be in the same order as the redox potentials, i.e. [Ru(4A-Cl-terpy)(bpy)O]2+ > [Ru(terpy)(bpy)O]2+ > [Ru(terpy)(4,4A-Me2-bpy)O]2+ > [Ru(terpy)(4,4A-EtO-bpy)O]2+.83 There has also been a study of the oxidation of guanines in DNA from calf thymu ...
... by [Ru(terpy)(bpy)O]2+ type complexes have been found to be in the same order as the redox potentials, i.e. [Ru(4A-Cl-terpy)(bpy)O]2+ > [Ru(terpy)(bpy)O]2+ > [Ru(terpy)(4,4A-Me2-bpy)O]2+ > [Ru(terpy)(4,4A-EtO-bpy)O]2+.83 There has also been a study of the oxidation of guanines in DNA from calf thymu ...
Appendix B: Agarose Physical Chemistry Agarose Physical
... One of the most important factors contributing to the success of agarose as an anticonvection medium is its ability to exhibit high gel strength at low concentrations (≤6%). Gel strength is defined as the force, expressed in g/cm2, that must be applied to fracture an agarose gel of a standard concen ...
... One of the most important factors contributing to the success of agarose as an anticonvection medium is its ability to exhibit high gel strength at low concentrations (≤6%). Gel strength is defined as the force, expressed in g/cm2, that must be applied to fracture an agarose gel of a standard concen ...
engineering chemistry
... An Atom is the smallest invisible particle of element, having all the characteristics of the parent element, which can neither be created nor destroyed by any chemical change. It cannot exist freely. It is the ultimate particle of an element, which may or may not have independent existence. The atom ...
... An Atom is the smallest invisible particle of element, having all the characteristics of the parent element, which can neither be created nor destroyed by any chemical change. It cannot exist freely. It is the ultimate particle of an element, which may or may not have independent existence. The atom ...