93102 Chemistry Scholarship exemplar-04
... weak base. As the titration is carried out and strong acid HCl is added, HOCH2CH2NH3+ and Cl– ions are formed. HOCH2CH2NH3+, the conjugate acid of a weak base, also dissociates in water to form H3O+ and HOCH2CH2NH2. HOCH2CH2NH3+ + H2O HOCH2CH2NH2 + H3O+. As more HCl is added, more HOCH2CH2NH3+ ions ...
... weak base. As the titration is carried out and strong acid HCl is added, HOCH2CH2NH3+ and Cl– ions are formed. HOCH2CH2NH3+, the conjugate acid of a weak base, also dissociates in water to form H3O+ and HOCH2CH2NH2. HOCH2CH2NH3+ + H2O HOCH2CH2NH2 + H3O+. As more HCl is added, more HOCH2CH2NH3+ ions ...
CHEMICAL EQUILIBRIUM
... PCl5 (g). after the system had reached equilibrium, 2.00 x 10 -3 mol Cl2 (g) was found in the flask. Gaseous PCl5 decomposes according to the following reaction: PCl5 (g) ↔ PCl3 (g) + Cl2 (g) Calculate the equilibrium concentrations of all species and the value of K. The expression for K is: ...
... PCl5 (g). after the system had reached equilibrium, 2.00 x 10 -3 mol Cl2 (g) was found in the flask. Gaseous PCl5 decomposes according to the following reaction: PCl5 (g) ↔ PCl3 (g) + Cl2 (g) Calculate the equilibrium concentrations of all species and the value of K. The expression for K is: ...
HYBRID MULTIDENTATE PHOSPHINE
... valuable support, guidance and encouragement being given by him during my PHD. It has been a true honour to work with Ian and I could not have asked for a better supervision, as he truly is an amazing individual beyond belief. Moreover, I also want to say my heartiest thanks to each and every person ...
... valuable support, guidance and encouragement being given by him during my PHD. It has been a true honour to work with Ian and I could not have asked for a better supervision, as he truly is an amazing individual beyond belief. Moreover, I also want to say my heartiest thanks to each and every person ...
water and aqueous solutions at high pressures and temperatures
... HDO and HO appear to become more alike, suggesting that dipole interactions other than those leading to hydrogen bonds become dominating in dense water above about 400°C. It is possible that the Raman spectrum of HDO diluted in normal water reveals more information about the hydrogen-bonded structur ...
... HDO and HO appear to become more alike, suggesting that dipole interactions other than those leading to hydrogen bonds become dominating in dense water above about 400°C. It is possible that the Raman spectrum of HDO diluted in normal water reveals more information about the hydrogen-bonded structur ...
- Opus
... acetylacetonate, Tl(I) acetylacetonate, Ba metal and dmaeH for unstable composite NiO/Tl2O3/BaO which on exposure to (2) in toluene to obtain crystalline products which were air is converted to NiO/Tl2O3/BaCO3 by the absorption of characterizes by physicochemical and spectroscopic methods. ...
... acetylacetonate, Tl(I) acetylacetonate, Ba metal and dmaeH for unstable composite NiO/Tl2O3/BaO which on exposure to (2) in toluene to obtain crystalline products which were air is converted to NiO/Tl2O3/BaCO3 by the absorption of characterizes by physicochemical and spectroscopic methods. ...
pyrimidin-7(4H)-one against Leishmani
... report their possible mechanism of action on L. infantum and L. braziliensis. Results: Antileishmanial effects are described for newly synthesized Cu(II) and Co(II) complexes containing 5methyl-1,2,4-triazolo[1,5-a]pyrimidin-7(4H)-one (HmtpO) as a ligand. These complexes display a wide structural di ...
... report their possible mechanism of action on L. infantum and L. braziliensis. Results: Antileishmanial effects are described for newly synthesized Cu(II) and Co(II) complexes containing 5methyl-1,2,4-triazolo[1,5-a]pyrimidin-7(4H)-one (HmtpO) as a ligand. These complexes display a wide structural di ...
1 of 11 Chem 481 Chapter 6 Answers to the Sixth Assignment Topic
... e) What is the most stable form of manganese in acid solution? In basic solution? How can you explain the difference? Mn2+ in acid, but Mn2O3 in base. From our study of Brønsted acidity, we recognize that Mn(III) cations are more acidic than Mn(II). In basic solution this leads to an oxide with a mu ...
... e) What is the most stable form of manganese in acid solution? In basic solution? How can you explain the difference? Mn2+ in acid, but Mn2O3 in base. From our study of Brønsted acidity, we recognize that Mn(III) cations are more acidic than Mn(II). In basic solution this leads to an oxide with a mu ...
Document
... 1. In the reaction 2 H2(g) + O2(g) → 2 H2O(l), 3 mol of gas-phase molecules is replaced by 2 mol of liquid-phase molecules, so ∆ng = −3 mol. Therefore, at 298 K, when RT = 2.5 kJ mol−1, the enthalpy and internal energy changes taking place in the system are related by • Note that the difference is e ...
... 1. In the reaction 2 H2(g) + O2(g) → 2 H2O(l), 3 mol of gas-phase molecules is replaced by 2 mol of liquid-phase molecules, so ∆ng = −3 mol. Therefore, at 298 K, when RT = 2.5 kJ mol−1, the enthalpy and internal energy changes taking place in the system are related by • Note that the difference is e ...
Chemistry - Swami Ramanand Teerth Marathwada University
... thermodynamics. Carnot’s cycle and its efficiency. Carnot’s theorem. d) Concept of entropy: Introduction, Definition, Mathematical Expression, Unit. Entropy as a state function. Entropy changes for reversible and irreversible processes in isolated systems. Entropy change in Physical transformations: ...
... thermodynamics. Carnot’s cycle and its efficiency. Carnot’s theorem. d) Concept of entropy: Introduction, Definition, Mathematical Expression, Unit. Entropy as a state function. Entropy changes for reversible and irreversible processes in isolated systems. Entropy change in Physical transformations: ...
Lab Manual - Center for Nonlinear Science
... Rules and Regulations is attached. You are required to read these and to acknowledge that you have read and understood them. Additionally, in the laboratory manuals/practical books and/or pre-practical lectures your attention will be drawn to the correct and safe handling of specific chemicals/reage ...
... Rules and Regulations is attached. You are required to read these and to acknowledge that you have read and understood them. Additionally, in the laboratory manuals/practical books and/or pre-practical lectures your attention will be drawn to the correct and safe handling of specific chemicals/reage ...
Coordination Chemistry Reviews On the medicinal chemistry of gold
... Other biological targets have also attracted attention. Auranofin and arsenic trioxide caused the inhibition of cellular selenoprotein synthesis, which indicates that gold agents may not only inhibit their main biological target TrxR directly but also perturb the related biochemistry concerning prot ...
... Other biological targets have also attracted attention. Auranofin and arsenic trioxide caused the inhibition of cellular selenoprotein synthesis, which indicates that gold agents may not only inhibit their main biological target TrxR directly but also perturb the related biochemistry concerning prot ...
Thermochemistry Exam Review Questions
... an unknown solution. What is the pH for the unknown solution likely to be? A. 1.2 B. 3.0 C. 5.3 D. 9.0 12. What is the name of the ion when a positively charged proton combines with a water molecule? A. ammonium ion B. hydrogen ion C. hydronium ion D. hydroxide ion 13. What is the term for a substan ...
... an unknown solution. What is the pH for the unknown solution likely to be? A. 1.2 B. 3.0 C. 5.3 D. 9.0 12. What is the name of the ion when a positively charged proton combines with a water molecule? A. ammonium ion B. hydrogen ion C. hydronium ion D. hydroxide ion 13. What is the term for a substan ...
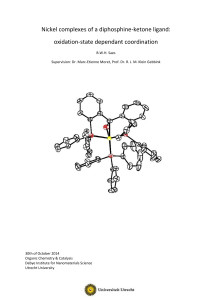
Nickel complexes of a diphosphine-ketone ligand: oxidation
... The requirement of chemically different activated hydrogen atoms also affects the hydrogen bond activation reaction type by the metal centred catalyst. Different H-H σ-bond activation types have been established[29,30], as can be seen in Figure 2. In the homolytic pathway ((A) in Figure 2), the two ...
... The requirement of chemically different activated hydrogen atoms also affects the hydrogen bond activation reaction type by the metal centred catalyst. Different H-H σ-bond activation types have been established[29,30], as can be seen in Figure 2. In the homolytic pathway ((A) in Figure 2), the two ...
Assignment 5 - Answers
... e) What is the most stable form of manganese in acid solution? In basic solution? How can you explain the difference? Mn2+ in acid, but Mn2O3 in base. From our study of Brønsted acidity, we recognize that Mn(III) cations are more acidic than Mn(II). In basic solution this leads to an oxide with a mu ...
... e) What is the most stable form of manganese in acid solution? In basic solution? How can you explain the difference? Mn2+ in acid, but Mn2O3 in base. From our study of Brønsted acidity, we recognize that Mn(III) cations are more acidic than Mn(II). In basic solution this leads to an oxide with a mu ...
Inorganic Chemistry of LanthanideIV:
... converters, for sensitizing photosensitive glasses and for polishing glass and stones. Ceria is also being used in infrared filters and as a replacement for radioactive thorium dioxide in incandescent mantles. While cerium dioxide is transparent for visible light, it is a strong ultraviolet light ab ...
... converters, for sensitizing photosensitive glasses and for polishing glass and stones. Ceria is also being used in infrared filters and as a replacement for radioactive thorium dioxide in incandescent mantles. While cerium dioxide is transparent for visible light, it is a strong ultraviolet light ab ...
Fall 2012
... 53. The vapor pressure for water is lower than expected for a polar substance with its molar mass because of which forces? a. ...
... 53. The vapor pressure for water is lower than expected for a polar substance with its molar mass because of which forces? a. ...
Hemilanthanidocene for the polymerization of styrene : from
... precatalysts or catalysts. A Cp*La[CH(SiMe3)2]2 (Cp* = C5Me5) half-lanthanocene was reported for the polymerization of various polar and non-polar monomers in a controlled fashion [1]. Cationic systems based on a (C5Me4SiMe3)Sc(CH2SiMe3)2(THF) half-scandocene were reported for the polymerization and ...
... precatalysts or catalysts. A Cp*La[CH(SiMe3)2]2 (Cp* = C5Me5) half-lanthanocene was reported for the polymerization of various polar and non-polar monomers in a controlled fashion [1]. Cationic systems based on a (C5Me4SiMe3)Sc(CH2SiMe3)2(THF) half-scandocene were reported for the polymerization and ...