Energetics - chemistryatdulwich
... and other reactions are not. The spontaneity or feasibility of a change (physical or chemical) depends on the interaction of two concepts, one of which was introduced in the SL course: Enthalpy: during a spontaneous change most systems show a tendency to release energy to take on a more stable low ...
... and other reactions are not. The spontaneity or feasibility of a change (physical or chemical) depends on the interaction of two concepts, one of which was introduced in the SL course: Enthalpy: during a spontaneous change most systems show a tendency to release energy to take on a more stable low ...
Transition metal sulfur chemistry and its relevance to
... The crystal structure of the nitrogenase proteins (ref. 12,19) has revealed the unique P and FeMoco metalloclusters and their arrangement in the protein. Recently, the complex of the iron protein with the iron molybdenum protein has also been crystallographicallyelucidated (ref. 20). It is beyond th ...
... The crystal structure of the nitrogenase proteins (ref. 12,19) has revealed the unique P and FeMoco metalloclusters and their arrangement in the protein. Recently, the complex of the iron protein with the iron molybdenum protein has also been crystallographicallyelucidated (ref. 20). It is beyond th ...
Therapeutic iron chelators and their potential side
... when bound by six oxygen atoms arranged octahedrally around the metal ion. A bidentate ligand occupies two of the above positions requiring three molecules t o totally encompass the iron atom. In contrast, all six coordination positions are occupied by a single hexadentate molecule. ...
... when bound by six oxygen atoms arranged octahedrally around the metal ion. A bidentate ligand occupies two of the above positions requiring three molecules t o totally encompass the iron atom. In contrast, all six coordination positions are occupied by a single hexadentate molecule. ...
Introduction to Organic Chemistry 2 ed William H. Brown
... Copyright © 2000 by John Wiley & Sons, Inc. All rights reserved. ...
... Copyright © 2000 by John Wiley & Sons, Inc. All rights reserved. ...
Structure of atoms
... A salt is an ionic compound that contains positively charged ions (often metal ions) and negatively charged ions. Sodium chloride, NaCl, is an example of a very common salt (called simply ‘salt’), but there are many other salts. Salts are formed (along with water) when acids react with bases during ...
... A salt is an ionic compound that contains positively charged ions (often metal ions) and negatively charged ions. Sodium chloride, NaCl, is an example of a very common salt (called simply ‘salt’), but there are many other salts. Salts are formed (along with water) when acids react with bases during ...
Cyclo-P3 Complexes of Vanadium: Redox
... complexes are prepared by activating white phosphorus (P4) with threecoordinate vanadium(II) precursors. Structural metrics, redox behavior, and DFT electronic structure analysis indicate that a [cyclo-P3]3− ligand is bound to a V(V) center in monomeric species 1 and 2. A salient feature of these ne ...
... complexes are prepared by activating white phosphorus (P4) with threecoordinate vanadium(II) precursors. Structural metrics, redox behavior, and DFT electronic structure analysis indicate that a [cyclo-P3]3− ligand is bound to a V(V) center in monomeric species 1 and 2. A salient feature of these ne ...
Document
... sulphide has a solubility of 70 mg.-moljm.3 (Weigel, cit. Seidell, 1920, p. 345). Its solubility in sea water is likely to have the same order of magnitude. Consequently, any amount of iron likely to be present in the foul bottom water of Loch Craiglin could be retained in solution as dissolved ferr ...
... sulphide has a solubility of 70 mg.-moljm.3 (Weigel, cit. Seidell, 1920, p. 345). Its solubility in sea water is likely to have the same order of magnitude. Consequently, any amount of iron likely to be present in the foul bottom water of Loch Craiglin could be retained in solution as dissolved ferr ...
...detail
... relations. (20 lectures) 3. Thermodynamics of Chemical Equilibrium Conditions of spontaneity and equilibrium. Degree of advancement and Le Chatelier principle; Van’t Hoff isotherm, isobar and isochore. (5 lectures) 4. Reaction Kinetics I Order and molecularity; determination of order (fractional lif ...
... relations. (20 lectures) 3. Thermodynamics of Chemical Equilibrium Conditions of spontaneity and equilibrium. Degree of advancement and Le Chatelier principle; Van’t Hoff isotherm, isobar and isochore. (5 lectures) 4. Reaction Kinetics I Order and molecularity; determination of order (fractional lif ...
1 [Turn Over Section A For each question there are four possible
... When iron filings are added to nitric acid, a yellow solution and nitrogen dioxide gas are formed. On the addition of ammonium thiocyanate to the resultant solution, a bloodred colouration due to an iron (III) complex is formed. Which statements are correct? ...
... When iron filings are added to nitric acid, a yellow solution and nitrogen dioxide gas are formed. On the addition of ammonium thiocyanate to the resultant solution, a bloodred colouration due to an iron (III) complex is formed. Which statements are correct? ...
COVER SHEET
... and optimised version of our procedure is described in the Experimental Section of this paper as a representative case of a general, high yield route to R2PCH2PR'2 ligand systems including 1 - 5 (Figure 1), which can easily be extended to a variety of other phosphine-containing molecules. ...
... and optimised version of our procedure is described in the Experimental Section of this paper as a representative case of a general, high yield route to R2PCH2PR'2 ligand systems including 1 - 5 (Figure 1), which can easily be extended to a variety of other phosphine-containing molecules. ...
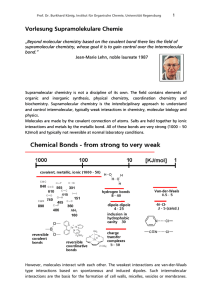
Vorlesung Supramolekulare Chemie
... The individual contributions to entropy loss in an aggregation or binding event are small, but they add up. If we now look at the enthalpies of covalent bonds, it is obvious that entropy does not play a significant role in the formation of bonds. Example: Reaction of two molecules forming one new C- ...
... The individual contributions to entropy loss in an aggregation or binding event are small, but they add up. If we now look at the enthalpies of covalent bonds, it is obvious that entropy does not play a significant role in the formation of bonds. Example: Reaction of two molecules forming one new C- ...
Supporting Information - Royal Society of Chemistry
... concentrations of inhibitors. For clarity, such plots are shown only for selected concentrations of inhibitors (Figure S3). The double reciprocal plots revealed that all inhibitors (complexes 2-4) were competitive types. However, since the concentration of the enzyme utilized during the above experi ...
... concentrations of inhibitors. For clarity, such plots are shown only for selected concentrations of inhibitors (Figure S3). The double reciprocal plots revealed that all inhibitors (complexes 2-4) were competitive types. However, since the concentration of the enzyme utilized during the above experi ...
Electrode Potentials
... Atoms of elements have no overall charge and are therefore given an oxidation number of zero. When two elements combine, the atoms or ions of the more electropositive element have a positive oxidation state, and those of the more electronegative element a negative oxidation state. Elements become mo ...
... Atoms of elements have no overall charge and are therefore given an oxidation number of zero. When two elements combine, the atoms or ions of the more electropositive element have a positive oxidation state, and those of the more electronegative element a negative oxidation state. Elements become mo ...
Trends in the Periodic Table I: Atomic and Ionic Radius
... by coordination compounds are stereoisomerism and structural isomerism. Stereoisomers include both geometric and optical isomers. Structural isomers include linkage, ionization, coordination, and hydrate isomers. The reactivities and properties of different isomers are often not the same and contrib ...
... by coordination compounds are stereoisomerism and structural isomerism. Stereoisomers include both geometric and optical isomers. Structural isomers include linkage, ionization, coordination, and hydrate isomers. The reactivities and properties of different isomers are often not the same and contrib ...