holiday home work - chemistry- xii-sc
... 9) The overall order of a reaction is 2. The units of the rate constant for the reaction are __________. A) M/s B) -1 -1 M s C) 1/s D) 1/M E) 2 s/M 10) The kinetics of the reaction below were studied and it was determined that the reaction rate increased by a factor of 9 when the concentration of B ...
... 9) The overall order of a reaction is 2. The units of the rate constant for the reaction are __________. A) M/s B) -1 -1 M s C) 1/s D) 1/M E) 2 s/M 10) The kinetics of the reaction below were studied and it was determined that the reaction rate increased by a factor of 9 when the concentration of B ...
info
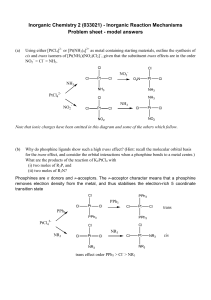
... arguments may be extrapolated to any other reaction generating a 16-electron from an 18-electron compound or any (n-2)electron from an n-electron compound. We will use CpMoX(PH3)2 as a model for the bis-PMe3 complexes previously used by us in several studies.7-11 The relevance of a sterics-related s ...
... arguments may be extrapolated to any other reaction generating a 16-electron from an 18-electron compound or any (n-2)electron from an n-electron compound. We will use CpMoX(PH3)2 as a model for the bis-PMe3 complexes previously used by us in several studies.7-11 The relevance of a sterics-related s ...
Receptor-assisted combinatorial chemistry
... that a combinatorial library must have to containing a ligand With this af?nity. The immune system has 107 to 108 mature B cells. When challenged for the ?rst time With a ligand (the antigen), this repertoire contains some antibodies that bind the antigen With disassociation constants in the range o ...
... that a combinatorial library must have to containing a ligand With this af?nity. The immune system has 107 to 108 mature B cells. When challenged for the ?rst time With a ligand (the antigen), this repertoire contains some antibodies that bind the antigen With disassociation constants in the range o ...
1.02 x 10 = 3 mol lit 3.4 x 10
... The colour of halogens is due to the fact that their molecules absorb radiations from visible light and the outer electrons are easily excited to higher energy levels. The amount of energy required for excitation depends upon the size of the atom. Fluorine atom is the smallest and the force of attra ...
... The colour of halogens is due to the fact that their molecules absorb radiations from visible light and the outer electrons are easily excited to higher energy levels. The amount of energy required for excitation depends upon the size of the atom. Fluorine atom is the smallest and the force of attra ...
Carbonyl-phosphine hetero-ligated half-metallocene iron(II
... catalysts are responsible not only for mediating initiation and propagation but also for molecular weight control, reaction rate and the range of applicable monomers, among other factors.2–5 For example, a catalyst (Mtn; n¼valence number) activates the carbon–halogen bond in an initiator (I–X) or in ...
... catalysts are responsible not only for mediating initiation and propagation but also for molecular weight control, reaction rate and the range of applicable monomers, among other factors.2–5 For example, a catalyst (Mtn; n¼valence number) activates the carbon–halogen bond in an initiator (I–X) or in ...
PDF File
... introduction, metal ion rescue experiments provide a powerful means to identify functional interactions and have been used extensively for protein and RNA enzymes. This approach has been extended to provide quantitative information that allows determination of whether substrate atoms are contacted b ...
... introduction, metal ion rescue experiments provide a powerful means to identify functional interactions and have been used extensively for protein and RNA enzymes. This approach has been extended to provide quantitative information that allows determination of whether substrate atoms are contacted b ...
IONIC EQULIBRIUM
... pH. The negative logarithm of the hydrogen ion concentration. pK. The negative logarithm of an equilibrium constant. Polyprotic acid. An acid which furnishes two or more protons. Range of an indicator. That portion of the pH scale over which an indicator changes color, roughly the pK of the indicato ...
... pH. The negative logarithm of the hydrogen ion concentration. pK. The negative logarithm of an equilibrium constant. Polyprotic acid. An acid which furnishes two or more protons. Range of an indicator. That portion of the pH scale over which an indicator changes color, roughly the pK of the indicato ...
SAMPLE EXAMINATION IV Section I – Multiple Choice
... Solubility equilibrium can be established by dissolving the solid, usually an ionic solid, into the solvent, usually water. This is generally regarded as the forward reaction. Solubility equilibrium can also be established by mixing solutions of ions that form a precipitate, in the reverse reaction. ...
... Solubility equilibrium can be established by dissolving the solid, usually an ionic solid, into the solvent, usually water. This is generally regarded as the forward reaction. Solubility equilibrium can also be established by mixing solutions of ions that form a precipitate, in the reverse reaction. ...
2008 Equilibrium -- without math (PowerPoint 13 MB)
... between forward and reverse reactions. In most cases, this balance is quite delicate. Changes in experimental conditions (concentration, pressure, volume and temperature) may disturb the balance and shift the equilibrium position so that more or less of the desired product is formed. When we say tha ...
... between forward and reverse reactions. In most cases, this balance is quite delicate. Changes in experimental conditions (concentration, pressure, volume and temperature) may disturb the balance and shift the equilibrium position so that more or less of the desired product is formed. When we say tha ...
A 1
... Which combinations of the three AOs are correct? The projection operator method provides a systematic way to find how the AOs should be combined to give the right group orbitals ...
... Which combinations of the three AOs are correct? The projection operator method provides a systematic way to find how the AOs should be combined to give the right group orbitals ...
Molarity Practice Worksheet
... the number of moles of solute divided by the number of liters of solution. In the first equation, the molarity will clearly be equal to 1.0 M, because there are 1.0 moles of NaCl and a solution volume of 1.0 L. In the second solution, the molarity will be different, because the solution volume will ...
... the number of moles of solute divided by the number of liters of solution. In the first equation, the molarity will clearly be equal to 1.0 M, because there are 1.0 moles of NaCl and a solution volume of 1.0 L. In the second solution, the molarity will be different, because the solution volume will ...
M.Sc - Veer Narmad South Gujarat University
... Reactivity of metal complexes, ligand replacement reaction: classification of mechanism and energy profile of reaction. Inert and labile complexes, interpretation of liability and inertness of transition metal complexes on the basis of VBT and CFT. Factors affecting the liability of a complex, trans ...
... Reactivity of metal complexes, ligand replacement reaction: classification of mechanism and energy profile of reaction. Inert and labile complexes, interpretation of liability and inertness of transition metal complexes on the basis of VBT and CFT. Factors affecting the liability of a complex, trans ...
IChO 2012
... Silicon hydrides SinH2n+2 are called silanes. Most of them contain Si–Si bonds, but they become increasingly unstable as the number of silicon atoms increases. a) Calculate the Si–Si bond dissociation enthalpy of Si 2H6 from the following information: Bond dissociation enthalpy for H–H = 436 kJ/mol ...
... Silicon hydrides SinH2n+2 are called silanes. Most of them contain Si–Si bonds, but they become increasingly unstable as the number of silicon atoms increases. a) Calculate the Si–Si bond dissociation enthalpy of Si 2H6 from the following information: Bond dissociation enthalpy for H–H = 436 kJ/mol ...
Chap15 - Bakersfield College
... Predicting the Direction of Reaction • How could we predict the direction in which a reaction at non-equilibrium conditions will shift to reestablish equilibrium? – To answer this question, substitute the current concentrations into the reaction quotient expression and compare it to Kc. – The react ...
... Predicting the Direction of Reaction • How could we predict the direction in which a reaction at non-equilibrium conditions will shift to reestablish equilibrium? – To answer this question, substitute the current concentrations into the reaction quotient expression and compare it to Kc. – The react ...
acids and bases - sukgr11chemistry
... meant . The statement nitric acid acts upon copper would be something more than mere words. All was still. In the interest of knowledge I was even willing to sacrifice one of the few copper cents then in my possession. I put one of them on the table, opened the bottle marked nitric acid, poured some ...
... meant . The statement nitric acid acts upon copper would be something more than mere words. All was still. In the interest of knowledge I was even willing to sacrifice one of the few copper cents then in my possession. I put one of them on the table, opened the bottle marked nitric acid, poured some ...
2E HARRY B. GRAY GEORGE S. HAMMONP.
... The material in this volume has been adapted primarily from a portion of the lectures given by H. B. 6. and 6. S. fl. to the Chemistry 2 students at the California Institute of Technology during the academic years 1966-1967 and 1967-1968. These lectures were taped, written up by J. B. D., and distri ...
... The material in this volume has been adapted primarily from a portion of the lectures given by H. B. 6. and 6. S. fl. to the Chemistry 2 students at the California Institute of Technology during the academic years 1966-1967 and 1967-1968. These lectures were taped, written up by J. B. D., and distri ...
Reactions of 1, 10-phenanthroline with hydrogen, lithium, sodium
... 1,10-phenanthroline exhibit absorption bands which are only slightly changed from the ligand bands. In cases like these it is convenient to determine sta bility constants if one can be assured that only two species the ligand and one of complexes AL^, exist in a given solu tion. This condition is ...
... 1,10-phenanthroline exhibit absorption bands which are only slightly changed from the ligand bands. In cases like these it is convenient to determine sta bility constants if one can be assured that only two species the ligand and one of complexes AL^, exist in a given solu tion. This condition is ...
a ΔG - KFUPM Resources v3
... system) is given by: ΔGrxn = Σn ΔGf (products) ‒ Σm ΔGf (reactants) where n and m are the stoichiometric coefficients of the reactants and products in a given equation. Dr. Al-Saadi ...
... system) is given by: ΔGrxn = Σn ΔGf (products) ‒ Σm ΔGf (reactants) where n and m are the stoichiometric coefficients of the reactants and products in a given equation. Dr. Al-Saadi ...