
Chemical fractionation at environmental interfaces
... mixture through differences in physical or chemical properties. In this thesis, it is occasionally used more broadly to mean partitioning, which emphasizes the separation of a species between different phases. An interface is a surface that forms the common boundary of two different phases. It can a ...
... mixture through differences in physical or chemical properties. In this thesis, it is occasionally used more broadly to mean partitioning, which emphasizes the separation of a species between different phases. An interface is a surface that forms the common boundary of two different phases. It can a ...
PDF (Chapter7)
... typical two-electron donors, substitution reactions of 7.7 with olefins were attempted. No reaction was observed by 1H or 31P{1H} NMR spectroscopy for the potentially η2-donors ethylene, 1-hexene, styrene, or azobenzene when present in THF solution in 1 to 10 equivalents. Examination of the literatu ...
... typical two-electron donors, substitution reactions of 7.7 with olefins were attempted. No reaction was observed by 1H or 31P{1H} NMR spectroscopy for the potentially η2-donors ethylene, 1-hexene, styrene, or azobenzene when present in THF solution in 1 to 10 equivalents. Examination of the literatu ...
chemistry-subject test5 w. solutions
... Dalton’s law of partial pressures states that the partial pressure of a gas in a mixture is equal to the total pressure of the mixture times the mole fraction. The mole fraction of gas A is the number of moles of A divided by the total number of moles of gases present (including A itself). If a mixt ...
... Dalton’s law of partial pressures states that the partial pressure of a gas in a mixture is equal to the total pressure of the mixture times the mole fraction. The mole fraction of gas A is the number of moles of A divided by the total number of moles of gases present (including A itself). If a mixt ...
Acids and Bases
... How can we identify an acid or a base simply by looking at the chemical formula? Since we have defined acids and bases by the ions they release in solution, the first requirement is that they contain H or OH, respectively. However, there are plenty of compounds that contain oxygen and hydrogen atoms t ...
... How can we identify an acid or a base simply by looking at the chemical formula? Since we have defined acids and bases by the ions they release in solution, the first requirement is that they contain H or OH, respectively. However, there are plenty of compounds that contain oxygen and hydrogen atoms t ...
Problem Set 4 Answers
... neutralization. What was the concentration of the sulfuric acid solution? 2 NaOH(aq) + H2SO4(aq) → Na2SO4 (aq) + 2 H2O(l) NaOH used = 16.12 x 10–3 L x 0.1021 mol.L–1 = 1.6459 x 10–3 mol Since one mole of sulfuric acid reacts with two moles of sodium hydroxide. mole NaOH = ...
... neutralization. What was the concentration of the sulfuric acid solution? 2 NaOH(aq) + H2SO4(aq) → Na2SO4 (aq) + 2 H2O(l) NaOH used = 16.12 x 10–3 L x 0.1021 mol.L–1 = 1.6459 x 10–3 mol Since one mole of sulfuric acid reacts with two moles of sodium hydroxide. mole NaOH = ...
View/Open
... (Section 11.2) in some respects. For example, they are able to form strong intermolecular hydrogen bonds, and therefore have higher boiling points than hydrocarbons of the same molecular weight. Phenol (bp 182 8C) has a boiling point more than 70 8C higher than toluene (bp 110.6 8C), even though th ...
... (Section 11.2) in some respects. For example, they are able to form strong intermolecular hydrogen bonds, and therefore have higher boiling points than hydrocarbons of the same molecular weight. Phenol (bp 182 8C) has a boiling point more than 70 8C higher than toluene (bp 110.6 8C), even though th ...
Comparison between Free and Immobilized Ion
... The Journal of Physical Chemistry B typical hydrophobicity of hydrocarbon molecules.23 The nanorod and nonpolar surface are solvated in a 2.5 nm × 2.5 nm × 6 nm water box (around 1100 water molecules). By carrying out two sets of simulations (with three counterions added or absent from the solutions ...
... The Journal of Physical Chemistry B typical hydrophobicity of hydrocarbon molecules.23 The nanorod and nonpolar surface are solvated in a 2.5 nm × 2.5 nm × 6 nm water box (around 1100 water molecules). By carrying out two sets of simulations (with three counterions added or absent from the solutions ...
Unit 9: ACIDS AND BASES
... 4. State whether each of the following statements is true or flase? a. The pH of a 0.10 mol dm-3 HCl is 2 b. The [H+] is a solution of pH = 3 is 100 times the [H+] in a solution of pH = 5 c. The [H+] in a solution of pH = 13 is 1.0 x 1013 mol dm-3 5. Calculate each of the following a. What is the pH ...
... 4. State whether each of the following statements is true or flase? a. The pH of a 0.10 mol dm-3 HCl is 2 b. The [H+] is a solution of pH = 3 is 100 times the [H+] in a solution of pH = 5 c. The [H+] in a solution of pH = 13 is 1.0 x 1013 mol dm-3 5. Calculate each of the following a. What is the pH ...
Determination of Equilibrium Constants for Reactions between Nitric
... outlet were measured by a gas analyzer (model CAI 650 NOXYGEN, which had a range of 3000 ppm and a repeatability of around 0.5% of full scale) from California Analytical Instruments Inc. Because the CAI 650 NOXYGEN analyzer could only work properly under atmospheric pressure, a “Tee” conjunction was ...
... outlet were measured by a gas analyzer (model CAI 650 NOXYGEN, which had a range of 3000 ppm and a repeatability of around 0.5% of full scale) from California Analytical Instruments Inc. Because the CAI 650 NOXYGEN analyzer could only work properly under atmospheric pressure, a “Tee” conjunction was ...
Equilibrium Booklet - mrstorie
... c) decreasing the pressure. d) decreasing the volume of the container. e) adding a solid drying agent such as CaCl2 which reacts with H2O(g). 3. For the reaction: 9.4 kJ + 2 HI(g) H2(g) + I2(g) a) What is the effect on [HI] if a small amount of H2 is added? b) What is the effect on [HI] if the pr ...
... c) decreasing the pressure. d) decreasing the volume of the container. e) adding a solid drying agent such as CaCl2 which reacts with H2O(g). 3. For the reaction: 9.4 kJ + 2 HI(g) H2(g) + I2(g) a) What is the effect on [HI] if a small amount of H2 is added? b) What is the effect on [HI] if the pr ...
Word Pro
... neutralization. What was the concentration of the sulfuric acid solution? 2 NaOH(aq) + H2SO4(aq) → Na2SO4 (aq) + 2 H2O(l) NaOH used = 16.12 x 10–3 L x 0.1021 mol.L–1 = 1.6459 x 10–3 mol Since one mole of sulfuric acid reacts with two moles of sodium hydroxide. ...
... neutralization. What was the concentration of the sulfuric acid solution? 2 NaOH(aq) + H2SO4(aq) → Na2SO4 (aq) + 2 H2O(l) NaOH used = 16.12 x 10–3 L x 0.1021 mol.L–1 = 1.6459 x 10–3 mol Since one mole of sulfuric acid reacts with two moles of sodium hydroxide. ...
Phosphorescent Organic Light-Emitting Devices: Working Principle and Iridium Based Emitter Materials
... smOLEDs). Because thin films of these emitter materials are usually prepared by vacuum deposition, the interest in organic electroluminescence has further been heightened by the description of electroluminescence from conjugated polymers providing the possibility of solution processing (frequently r ...
... smOLEDs). Because thin films of these emitter materials are usually prepared by vacuum deposition, the interest in organic electroluminescence has further been heightened by the description of electroluminescence from conjugated polymers providing the possibility of solution processing (frequently r ...
* Porphyrins. XXVII.
... band rather than any intensity redistribution among Q, B, and N bands. The Pb(IV) spectra do not seem to have previously been reported. As discussed in Sec. II B, Pb(IV)OE P was made but was only stable at low temperature. An excitation and an emission spec- ...
... band rather than any intensity redistribution among Q, B, and N bands. The Pb(IV) spectra do not seem to have previously been reported. As discussed in Sec. II B, Pb(IV)OE P was made but was only stable at low temperature. An excitation and an emission spec- ...
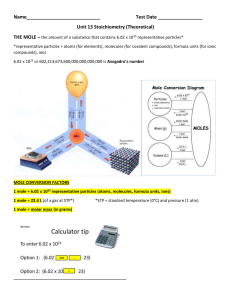
Unit 13 Stoichiometry (Theoretical)
... Ionic compounds o In 1 mole of Ca(NO3)2 there are 1 mole of calcium ions and 2 moles of nitrate ions o In 1 mole of K2O, the ion ratio is 2 mole K+1 ions: 1 mole of O-2 ions ...
... Ionic compounds o In 1 mole of Ca(NO3)2 there are 1 mole of calcium ions and 2 moles of nitrate ions o In 1 mole of K2O, the ion ratio is 2 mole K+1 ions: 1 mole of O-2 ions ...
Association of Nitrate Ion with Metal Cations in Aqueous Solution: a
... (k = 1, 2, ...). The estimated number of principal components, K, is usually called the pseudorank or effective rank of A. In the present context, it is interpreted as the number of spectrally distinguishable chemical species. Evolving Factor Analysis (EFA) The unknown matrices E and C cannot be com ...
... (k = 1, 2, ...). The estimated number of principal components, K, is usually called the pseudorank or effective rank of A. In the present context, it is interpreted as the number of spectrally distinguishable chemical species. Evolving Factor Analysis (EFA) The unknown matrices E and C cannot be com ...
PAGE PROOFS
... where it is used to help break down food. It is also used in industry, where it is sometimes called ‘spirit of salts’, to clean bricks and to clean off the coating of oxide on corroded iron or steel before plating the metal with a protective layer of zinc or tin. Table 13.1 (see page 000) lists comm ...
... where it is used to help break down food. It is also used in industry, where it is sometimes called ‘spirit of salts’, to clean bricks and to clean off the coating of oxide on corroded iron or steel before plating the metal with a protective layer of zinc or tin. Table 13.1 (see page 000) lists comm ...
Preparation and Inner-sphere Oxidation of Ternary Iminodiacetato Chromium [III]
... for CrIII, Su, IDA and HNO3 at 25ºC and ionic strength 0.1 mol dm-3. The addition of CrIII to the free ligands solution shifts the buffer region of the ligand to lower pH values. This indicates that the formation of the complex proceed via releasing of protons from such ligands. From curve 6 in figu ...
... for CrIII, Su, IDA and HNO3 at 25ºC and ionic strength 0.1 mol dm-3. The addition of CrIII to the free ligands solution shifts the buffer region of the ligand to lower pH values. This indicates that the formation of the complex proceed via releasing of protons from such ligands. From curve 6 in figu ...
Periodic Trends in Monoatomic Chemisorbate
... The most versatile tactic involves the electrodeposition of ultrathin (2-5 monolayer, ML) transition-metal films onto a SERS-active gold substrate. Such films, which impart nearoptimal SERS activity to adsorbates on the overlayer metal, have been employed extensively in our laboratory to examine che ...
... The most versatile tactic involves the electrodeposition of ultrathin (2-5 monolayer, ML) transition-metal films onto a SERS-active gold substrate. Such films, which impart nearoptimal SERS activity to adsorbates on the overlayer metal, have been employed extensively in our laboratory to examine che ...





















![Preparation and Inner-sphere Oxidation of Ternary Iminodiacetato Chromium [III]](http://s1.studyres.com/store/data/008844767_1-9b02a033035d53dea970333df8a85c48-300x300.png)
