COMPLEXES
... strong metal-ligand bond. The complexes are insoluble in common organic solvents such as ethanol, acetone, etc. but are fairly soluble in methanol, chloroform, DMF and DMSO. The elemental analysis data (Table 2) of metal complexes is consistent with their general formulation as 1:1:1, mixed ligand c ...
... strong metal-ligand bond. The complexes are insoluble in common organic solvents such as ethanol, acetone, etc. but are fairly soluble in methanol, chloroform, DMF and DMSO. The elemental analysis data (Table 2) of metal complexes is consistent with their general formulation as 1:1:1, mixed ligand c ...
South Pasadena • AP Chemistry
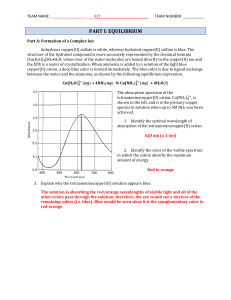
... 7. What does it mean when an equilibrium system is described as a dynamic system? Explain. Dynamic is a term that refers to a process that never stops – the forward process & reverse processes occur at the same rate giving the appearance that the process has stopped, but that is NOT the case. 8. Dra ...
... 7. What does it mean when an equilibrium system is described as a dynamic system? Explain. Dynamic is a term that refers to a process that never stops – the forward process & reverse processes occur at the same rate giving the appearance that the process has stopped, but that is NOT the case. 8. Dra ...
IOSR Journal of Applied Chemistry (IOSR-JAC) e-ISSN: 2278-5736.
... was added drop wise to the above solution. The reaction mixture was refluxed for 3 h. and filtered. The filtrate was concentrated and was left overnight at room temperature.The solid mass was filtered, washed repeatedly with water.The product so obtained was washed with methanol, diethyl ether and d ...
... was added drop wise to the above solution. The reaction mixture was refluxed for 3 h. and filtered. The filtrate was concentrated and was left overnight at room temperature.The solid mass was filtered, washed repeatedly with water.The product so obtained was washed with methanol, diethyl ether and d ...
Chapter 4 - GEOCITIES.ws
... Electricity is moving charges. Electrolytic solutions have the ability to conduct electricity. The ions that are dissolved can move. Solutions of ionic compounds can conduct electricity. (called electrolytic solution) Ionic solids dissociate into it’s component ions as it dissolves ...
... Electricity is moving charges. Electrolytic solutions have the ability to conduct electricity. The ions that are dissolved can move. Solutions of ionic compounds can conduct electricity. (called electrolytic solution) Ionic solids dissociate into it’s component ions as it dissolves ...
Electronic spectroscopy • Lecture 5: π
... Summary of Course – week 5 Complexes of π-acceptor ligands • be able to explain synergic (σ-donation, π-back donation) model for bonding in M-CO and M-N2 complexes • be able to explain reduction in CO stretching frequency in complex • be able to explain changes in CO stretching frequency with metal ...
... Summary of Course – week 5 Complexes of π-acceptor ligands • be able to explain synergic (σ-donation, π-back donation) model for bonding in M-CO and M-N2 complexes • be able to explain reduction in CO stretching frequency in complex • be able to explain changes in CO stretching frequency with metal ...
- LSU Chemistry
... classes and teaching concepts learned in this and other chemistry classes (General Chemistry). You will enhance your teaching efforts by doing a set of demonstrations that illustrate the concepts being taught. There is a 2-4 page typed report (reflection essay) required describing your teaching expe ...
... classes and teaching concepts learned in this and other chemistry classes (General Chemistry). You will enhance your teaching efforts by doing a set of demonstrations that illustrate the concepts being taught. There is a 2-4 page typed report (reflection essay) required describing your teaching expe ...
V α - Springer
... Postulates of thermodynamics 1. There exist particular states (called equilibrium states) of simple systems that, macroscopically, are characterized completely by the internal energy U, the volume V, and the amounts of the K chemical components n1, n2,…, nK . 2. There exists a function (called the ...
... Postulates of thermodynamics 1. There exist particular states (called equilibrium states) of simple systems that, macroscopically, are characterized completely by the internal energy U, the volume V, and the amounts of the K chemical components n1, n2,…, nK . 2. There exists a function (called the ...
Document
... Molecular equation: balanced chemical equation showing neutral formulas for all reactants and products. Ca(NO3)2(aq) + Na2SO4(aq) → CaSO4(s) + 2 NaNO3(aq) Complete ionic equation: all strong electrolytes are written as separate ions with their own coefficients and phase labels. (Pure solids, liquids ...
... Molecular equation: balanced chemical equation showing neutral formulas for all reactants and products. Ca(NO3)2(aq) + Na2SO4(aq) → CaSO4(s) + 2 NaNO3(aq) Complete ionic equation: all strong electrolytes are written as separate ions with their own coefficients and phase labels. (Pure solids, liquids ...
Substituent Effect in the γ–Position of Acetylacetonate on the
... of Cl-acac chelate makes the coordination sphere around the metal ion electron-poor, which makes it easier for solvent molecules to approach the axial centre and accordingly, leading to a greater solvatochromic effect. As evident from Figure 1 and Table 1, the νmax values in one particular solvent d ...
... of Cl-acac chelate makes the coordination sphere around the metal ion electron-poor, which makes it easier for solvent molecules to approach the axial centre and accordingly, leading to a greater solvatochromic effect. As evident from Figure 1 and Table 1, the νmax values in one particular solvent d ...
Uranyl Ion Complexes with Ammoniobenzoates as
... atoms being thus in pentagonal bipyramidal environments. The average U–O bond lengths for oxo, hydroxo, bridging carboxylate, chelating carboxylate and water ligands are unexceptional, at 2.27(4), 2.34(5), 2.39(6), 2.495(13) and 2.50(6) Å, respectively. Although this tetranuclear motif is very close ...
... atoms being thus in pentagonal bipyramidal environments. The average U–O bond lengths for oxo, hydroxo, bridging carboxylate, chelating carboxylate and water ligands are unexceptional, at 2.27(4), 2.34(5), 2.39(6), 2.495(13) and 2.50(6) Å, respectively. Although this tetranuclear motif is very close ...
Metal / σ-bond interactions
... to explain these phenomena, another type of metathesis mechanism was invented. ...
... to explain these phenomena, another type of metathesis mechanism was invented. ...
Preparation and Characterization of Nickel and
... When the ligands can bind in only one way, the coordination number of the complex can be determined from the formula. NH3 and similar ligands can only be monodentate, o-phenanthroline can only be bidentate, and so forth. But some ligands can bond in more than one way. For example, NO3- can be uncoor ...
... When the ligands can bind in only one way, the coordination number of the complex can be determined from the formula. NH3 and similar ligands can only be monodentate, o-phenanthroline can only be bidentate, and so forth. But some ligands can bond in more than one way. For example, NO3- can be uncoor ...
Chapter 1 - DORAS
... undergo more efficient supramolecular processes. This substitution may have the affect of altering the energy gap between the excited state (3MLCT) and the deactivation pathways within the system. The photo-redox properties of [Ru(bpy)3]2+ also generates interest. Normally oxidation of [Ru(bpy)3]2+ ...
... undergo more efficient supramolecular processes. This substitution may have the affect of altering the energy gap between the excited state (3MLCT) and the deactivation pathways within the system. The photo-redox properties of [Ru(bpy)3]2+ also generates interest. Normally oxidation of [Ru(bpy)3]2+ ...
Pentadienyl Complexes of Alkali Metals
... obtain for the metal–ligand interaction energy. The relative energy between 7 and 2 is useful, since 7 is not connected directly to 1. Note that the latter energy differences increase gradually from Li to Rb. In the case of the Cs complex, this barrier is slightly lower than that in the Rb compound. ...
... obtain for the metal–ligand interaction energy. The relative energy between 7 and 2 is useful, since 7 is not connected directly to 1. Note that the latter energy differences increase gradually from Li to Rb. In the case of the Cs complex, this barrier is slightly lower than that in the Rb compound. ...