THE GENERAL FEATURES OF TRANSITION METAL CHEMISTRY
... The real explanation is going to be much more difficult than it seems at first sight. Neither can you use the statement that a full d level (for example, in the copper case) is stable, unless you can come up with a proper explanation of why that is. You can't assume that looking nice and tidy is ...
... The real explanation is going to be much more difficult than it seems at first sight. Neither can you use the statement that a full d level (for example, in the copper case) is stable, unless you can come up with a proper explanation of why that is. You can't assume that looking nice and tidy is ...
solutions can`t be made accurately by weighing the solid, must be
... the conditions (in the car battery) are not standard (b) (i) When a metal is placed in a solution of its ions, the electrical potential set up between the metal and the solution cannot be measured without using a reference electrode. Explain why this is so. (ii) Label the diagram of the standard hyd ...
... the conditions (in the car battery) are not standard (b) (i) When a metal is placed in a solution of its ions, the electrical potential set up between the metal and the solution cannot be measured without using a reference electrode. Explain why this is so. (ii) Label the diagram of the standard hyd ...
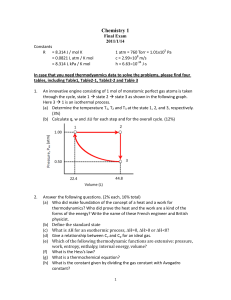
CHEM230P1_06_2014_Y_P1
... A 1.0504 g sample of titanium ore was treated with hydrogen peroxide and 1 M sulfuric acid to form a coloured complex. The final volume of the solution was 100.00 mL. A 1.00 mL aliquot of this solution was diluted to 250.00 mL and the absorbance of this diluted solutio ...
... A 1.0504 g sample of titanium ore was treated with hydrogen peroxide and 1 M sulfuric acid to form a coloured complex. The final volume of the solution was 100.00 mL. A 1.00 mL aliquot of this solution was diluted to 250.00 mL and the absorbance of this diluted solutio ...
K b
... bases are added or when dilution occurs. • The buffer is a mixture of an acid and its conjugate base. There must be comparable amounts of the conjugate acid and base (say, within a factor of 10) to exert significant buffering. ...
... bases are added or when dilution occurs. • The buffer is a mixture of an acid and its conjugate base. There must be comparable amounts of the conjugate acid and base (say, within a factor of 10) to exert significant buffering. ...
Fp*) Fe(η5-C5Me5)(CO)2
... and 1.36 Å, respectively. Although the CtC lengths are comparable to those in 5 and 6, the C-C distances are slightly shorter than those in 6 (by 0.03 Å). In contrast to the similarity in the (CtC)n parts, the Fe-C lengths of 1 (averaged value: 1.88 Å) are substantially shorter than those in 5 and 6 ...
... and 1.36 Å, respectively. Although the CtC lengths are comparable to those in 5 and 6, the C-C distances are slightly shorter than those in 6 (by 0.03 Å). In contrast to the similarity in the (CtC)n parts, the Fe-C lengths of 1 (averaged value: 1.88 Å) are substantially shorter than those in 5 and 6 ...
Reactions of Non-Metals
... Nonmetal oxides react with water to form acids: SO3 (g) + H2O (ℓ) Ý H2SO4 (ℓ) N2O5 (s) + H2O (ℓ) Ý 2 HNO3 (ℓ) N2O3 (g) + H2O (ℓ) Ý 2 HNO2 (aq) P4O10 (s) + 6 H2O (ℓ) Ý 4 H3PO4 (s) In the reverse reactions, oxoacids decompose into the nonmetal oxide and water. For these reactions, the oxidation number ...
... Nonmetal oxides react with water to form acids: SO3 (g) + H2O (ℓ) Ý H2SO4 (ℓ) N2O5 (s) + H2O (ℓ) Ý 2 HNO3 (ℓ) N2O3 (g) + H2O (ℓ) Ý 2 HNO2 (aq) P4O10 (s) + 6 H2O (ℓ) Ý 4 H3PO4 (s) In the reverse reactions, oxoacids decompose into the nonmetal oxide and water. For these reactions, the oxidation number ...
(b) Costes, J - Nanyang Technological University
... latter, two different interactions may occur: Co-Er and ErEr. Thus, the magnetic behavior of the latter species corresponds not only to the Co-Er interaction but also to the overall behavior of the molecule, including both interactions as well as crystal field effects. Therefore, more detailed exper ...
... latter, two different interactions may occur: Co-Er and ErEr. Thus, the magnetic behavior of the latter species corresponds not only to the Co-Er interaction but also to the overall behavior of the molecule, including both interactions as well as crystal field effects. Therefore, more detailed exper ...
UNIVERSITY OF THE WEST INDIES MONA, JAMAICA
... octahedral (1/2 occupied) sites in the lattice, giving a Unit Cell with 8 Mg's, 16 Al's and 32 O's. An inverse spinel is an alternative arrangement where half of the trivalent ions swap with the divalent ions so that the M(II) now occupy octahedral sites i.e. B(AB)O4. OSPE calculations are often abl ...
... octahedral (1/2 occupied) sites in the lattice, giving a Unit Cell with 8 Mg's, 16 Al's and 32 O's. An inverse spinel is an alternative arrangement where half of the trivalent ions swap with the divalent ions so that the M(II) now occupy octahedral sites i.e. B(AB)O4. OSPE calculations are often abl ...
CHEMICAL REACTIONS
... e. Lewis acid (e- pr acceptor) and Lewis base (e- pr. donor) • e.g. boron trifluoride (e- deficient) and ammonia (lone pair on N) : BF3 + NH3 Æ BF3NH3 B. Decomposition/ Analysis Reactions 1. decomposition (not necessarily heated): a. hydrogen peroxide decomposes into water and oxygen. b. ammonium hy ...
... e. Lewis acid (e- pr acceptor) and Lewis base (e- pr. donor) • e.g. boron trifluoride (e- deficient) and ammonia (lone pair on N) : BF3 + NH3 Æ BF3NH3 B. Decomposition/ Analysis Reactions 1. decomposition (not necessarily heated): a. hydrogen peroxide decomposes into water and oxygen. b. ammonium hy ...
Chemistry 3211 - Inorganic Chemistry of the Transition Metals
... numbers; catalytic reactions of organometallic species (parts of Ch 26). ...
... numbers; catalytic reactions of organometallic species (parts of Ch 26). ...
Document
... • When ionic compounds dissolve in water, the anions and cations are separated from each other. This is called dissociation. – However, not all ionic compounds are soluble in water! • When compounds containing polyatomic ions dissociate, the polyatomic group stays together as one ion. • When molecul ...
... • When ionic compounds dissolve in water, the anions and cations are separated from each other. This is called dissociation. – However, not all ionic compounds are soluble in water! • When compounds containing polyatomic ions dissociate, the polyatomic group stays together as one ion. • When molecul ...
2 - Personal WWW Pages
... (which is greater for P, S and Se donors). It should be noted that the effect of the paramagnetic shielding term is not only restricted to quadrupolar nuclei. Any species with low energy paramagnetic excited states can exhibit this effect. Thus 103Rh (I = ½ ) also has a very wide chemical shift rang ...
... (which is greater for P, S and Se donors). It should be noted that the effect of the paramagnetic shielding term is not only restricted to quadrupolar nuclei. Any species with low energy paramagnetic excited states can exhibit this effect. Thus 103Rh (I = ½ ) also has a very wide chemical shift rang ...
HL Periodicity
... As the d-block fills, there are electrons available for other functions (create colors). The atomic radii are curious, also. Based on electron config. ...
... As the d-block fills, there are electrons available for other functions (create colors). The atomic radii are curious, also. Based on electron config. ...
SCH4C Exam Review Assignment Kathleen Fall 2014
... Unit 2: Quantities in Chemistry 1. Calculate the molar mass of the following molecules or formula units: b) Ba(NO3)2 c) K3PO4 a) C6H6 ...
... Unit 2: Quantities in Chemistry 1. Calculate the molar mass of the following molecules or formula units: b) Ba(NO3)2 c) K3PO4 a) C6H6 ...
THE ELECTROCHEMISTRY OF STRAPPED AND CAPPED PORPHYRIN
... The preparation of 'IT-cation radicals of porphyrinic derivatIves by photochemical [5], chemical [6] and electrochemical methods [7] is well known. The latter two methods also permit the oxidation of the porphyrin ring (P) to generate dicationic species from both free bases and divalent metal ion co ...
... The preparation of 'IT-cation radicals of porphyrinic derivatIves by photochemical [5], chemical [6] and electrochemical methods [7] is well known. The latter two methods also permit the oxidation of the porphyrin ring (P) to generate dicationic species from both free bases and divalent metal ion co ...
Lecture Sections 15.6 - Fulton County Schools
... 1. Experimentally determined solubility of an ionic solid can be used to calculate its Ksp value. 2. Solubility of an ionic solid can be calculated if its Ksp value is known. 3. Relative solubilities can be predicted by comparing Ksp values only for salts that produce the same total # of ions. 4. So ...
... 1. Experimentally determined solubility of an ionic solid can be used to calculate its Ksp value. 2. Solubility of an ionic solid can be calculated if its Ksp value is known. 3. Relative solubilities can be predicted by comparing Ksp values only for salts that produce the same total # of ions. 4. So ...
Brønsted acid
... water, results in a solution that can conduct electricity. A nonelectrolyte is a substance that, when dissolved, results in a solution that does not conduct electricity. ...
... water, results in a solution that can conduct electricity. A nonelectrolyte is a substance that, when dissolved, results in a solution that does not conduct electricity. ...
this PDF file
... reaction. However it is not easy to accurately calculate and plot the standard free energy changes and equilibrium constants for reactions due to the calculation complexity of reactions and phase transitions. It is found in the literature (Li, 2001) that it is not simple and convenient for calculati ...
... reaction. However it is not easy to accurately calculate and plot the standard free energy changes and equilibrium constants for reactions due to the calculation complexity of reactions and phase transitions. It is found in the literature (Li, 2001) that it is not simple and convenient for calculati ...
Acids, Bases, and Buffers
... The function of a buffer can be examined using LeChatelier’s Principle. When a strong acid is added to a buffer solution it ionizes completely forming H3O+. The H3O+ produced from the strong acid becomes part of the equilibrium. The concentration of H3O+ in the equilibrium reaction has been increase ...
... The function of a buffer can be examined using LeChatelier’s Principle. When a strong acid is added to a buffer solution it ionizes completely forming H3O+. The H3O+ produced from the strong acid becomes part of the equilibrium. The concentration of H3O+ in the equilibrium reaction has been increase ...