Metal Complexes as Antimicrobial Agents
... and Grisofluvine were used as reference compounds for antbacterial and antifungal activities, respectively. Escherichia coli, Salmonella typhi, Staphylococcus aureus and Bacillus subtillus (bacteria) or Aspergillus terreus and Aspergillus flavus (Fungi) were used as the test organisms. Results have ...
... and Grisofluvine were used as reference compounds for antbacterial and antifungal activities, respectively. Escherichia coli, Salmonella typhi, Staphylococcus aureus and Bacillus subtillus (bacteria) or Aspergillus terreus and Aspergillus flavus (Fungi) were used as the test organisms. Results have ...
Document
... of 2 and 4. In the past, ESI-MS has proved invaluable for qualitative and quantitative evaluation of host binding selectivities in weakly bound, noncovalent host-guest complexes in the gas phase.9-21 ESI-MS is quite useful for obtaining an initial assessment of the metal binding affinities of new li ...
... of 2 and 4. In the past, ESI-MS has proved invaluable for qualitative and quantitative evaluation of host binding selectivities in weakly bound, noncovalent host-guest complexes in the gas phase.9-21 ESI-MS is quite useful for obtaining an initial assessment of the metal binding affinities of new li ...
Oxidation Numbers and Writing Redox Equations
... (a) write the redox half reaction equation for the formation of Fe3+(aq) ions (b) derive and write the redox half reaction equation for the conversion of MnO4-(aq) ions to Mn2+(aq) ions (c) combine the two redox half reaction equations to write the balanced ionic equation for the reaction between Fe ...
... (a) write the redox half reaction equation for the formation of Fe3+(aq) ions (b) derive and write the redox half reaction equation for the conversion of MnO4-(aq) ions to Mn2+(aq) ions (c) combine the two redox half reaction equations to write the balanced ionic equation for the reaction between Fe ...
Webquest Review - Harrison High School
... CO2 is nonpolar. You have two oxygens, each double bonded to the carbon. These two identical bonds in a symmetrical species, pull in equal yet opposite directions, cancelling each other out. E: View “Intermolecular Forces.” 20. Explain why magnesium chloride should dissolve in water. Magnesium chlor ...
... CO2 is nonpolar. You have two oxygens, each double bonded to the carbon. These two identical bonds in a symmetrical species, pull in equal yet opposite directions, cancelling each other out. E: View “Intermolecular Forces.” 20. Explain why magnesium chloride should dissolve in water. Magnesium chlor ...
I- Introduction
... The Scope of Analytical Chemistry: Analytical chemistry has bounds which are amongst the widest of any technological discipline. An analyst must be able to design, carry out, and interpret his measurements within the context of the fundamental technological problem with which he is presented. The se ...
... The Scope of Analytical Chemistry: Analytical chemistry has bounds which are amongst the widest of any technological discipline. An analyst must be able to design, carry out, and interpret his measurements within the context of the fundamental technological problem with which he is presented. The se ...
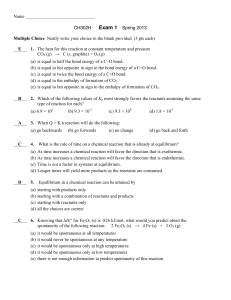
Exam 1 Key
... 6. Knowing that ∆Hf° for Fe2O3 (s) is -826 kJ/mol, what would you predict about the spontaneity of the following reaction: 2 Fe2O3 (s) → 4 Fe (s) + 3 O2 (g) (a) ...
... 6. Knowing that ∆Hf° for Fe2O3 (s) is -826 kJ/mol, what would you predict about the spontaneity of the following reaction: 2 Fe2O3 (s) → 4 Fe (s) + 3 O2 (g) (a) ...
Aqueous Reactions and Solution Stoichiometry (Chapter 4)
... equivalence point is reached. The equivalence point is reached when stoichiometrically equal amounts of each reactant have been added to the solution. In most titrations, if a suitable indicator (a substance that changes color at a specific color) is used, the indicator will change color (the end-po ...
... equivalence point is reached. The equivalence point is reached when stoichiometrically equal amounts of each reactant have been added to the solution. In most titrations, if a suitable indicator (a substance that changes color at a specific color) is used, the indicator will change color (the end-po ...
Get Day 16 - Mattson Creighton
... attempting to predict the splitting pattern for other geometries, two important tools make the job easier. 1. The character table lets you know which of the d-orbitals are transform the same (have the same symmetry) and are thus degenerate. 2. The d-orbitals can be sketched into a Cartesian coordina ...
... attempting to predict the splitting pattern for other geometries, two important tools make the job easier. 1. The character table lets you know which of the d-orbitals are transform the same (have the same symmetry) and are thus degenerate. 2. The d-orbitals can be sketched into a Cartesian coordina ...
UNSYMMETRICAL DINUCLEAR RHODIUM COMPLEXES WITH
... atom and one or more sulfur atoms in the same ligand is not widely exploited. Catalytic applications may be complicated by the fact that sulfur species are considered to be potential catalyst poisons that cause a substantial decrease in the catalytic activity.16 However, promotional rather than pois ...
... atom and one or more sulfur atoms in the same ligand is not widely exploited. Catalytic applications may be complicated by the fact that sulfur species are considered to be potential catalyst poisons that cause a substantial decrease in the catalytic activity.16 However, promotional rather than pois ...
Title Iridium, ruthenium, and palladium complexes containing a
... ligand precursor was recovered. Despite extensive efforts to purify complex 4, we did not succeed in obtaining microanalytically pure material. Purification was thwarted by the formation of palladium black during this process, which may point towards a limited stability of complex 4 or the anticipat ...
... ligand precursor was recovered. Despite extensive efforts to purify complex 4, we did not succeed in obtaining microanalytically pure material. Purification was thwarted by the formation of palladium black during this process, which may point towards a limited stability of complex 4 or the anticipat ...
Additional questions
... Concentrated sulfuric acid contains very little water, only 5.0% by mass. It has a density of 1.84 g/mL. What is the molarity of this acid? ...
... Concentrated sulfuric acid contains very little water, only 5.0% by mass. It has a density of 1.84 g/mL. What is the molarity of this acid? ...
σ−Bonded ligands: Transition Metal Alkyls and Hydrides
... Where β elimination cannot occur for the reasons discussed above, α elimination sometimes takes over. ...
... Where β elimination cannot occur for the reasons discussed above, α elimination sometimes takes over. ...
Introduction to Qualitative Analysis
... It is important to recognize the distinction between these groups and the groups of the Periodic Table (alkali metals, transition metals, etc.); the groups A-D do not necessarily correlate with groups in the Periodic Table. Periodic Table groups are based upon similarities in electron configurations ...
... It is important to recognize the distinction between these groups and the groups of the Periodic Table (alkali metals, transition metals, etc.); the groups A-D do not necessarily correlate with groups in the Periodic Table. Periodic Table groups are based upon similarities in electron configurations ...
Synthesis and Structure of Six-Coordinate Iron Borohydride
... iron complexes supported by bifunctional tetradentate PNNPtype ligands as highly efficient catalysts for the transfer hydrogenation of ketones,5 while Milstein and co-workers reported that pincer complexes such as {2,6-C 5 H 3 N(CH2PiPr2)2}FeH(η1-HBH3)(CO) are active catalysts for the base-free hydrog ...
... iron complexes supported by bifunctional tetradentate PNNPtype ligands as highly efficient catalysts for the transfer hydrogenation of ketones,5 while Milstein and co-workers reported that pincer complexes such as {2,6-C 5 H 3 N(CH2PiPr2)2}FeH(η1-HBH3)(CO) are active catalysts for the base-free hydrog ...
PS_CHEM7_ch4 - WordPress.com
... purifying sewage. In aqueous solution, it reacts with base to form a white precipitate. (a) Write balanced total and net ionic equations for its reaction with aqueous NaOH. (b) What mass of precipitate forms when185.5 mL of 0.533 M NaOH is added to 627 mL of a solution that contains 15.8 g of alumin ...
... purifying sewage. In aqueous solution, it reacts with base to form a white precipitate. (a) Write balanced total and net ionic equations for its reaction with aqueous NaOH. (b) What mass of precipitate forms when185.5 mL of 0.533 M NaOH is added to 627 mL of a solution that contains 15.8 g of alumin ...
Questions 1-2
... 18. The phase diagram for the pure substance X is shown above. The temperature of a sample of pure solid X is slowly raised from 10°C to 100°C at a constant pressure of 0.5 atm. What is the expected behavior of the substance? (A) It first melts to a liquid and then boils at about 70°(C) (B) It first ...
... 18. The phase diagram for the pure substance X is shown above. The temperature of a sample of pure solid X is slowly raised from 10°C to 100°C at a constant pressure of 0.5 atm. What is the expected behavior of the substance? (A) It first melts to a liquid and then boils at about 70°(C) (B) It first ...
- Gondwana University, Gadchiroli
... (A) Second law of thermodynamics : Need for second law of thermodynamics, statements of second law of thermodynamics, concept of entropy, entropy as a state function of V & T,P&T, entropy change in phase change for ideal gas, entropy as criteria of spontaneity & equilibrium. [4L] (B) Free energy fun ...
... (A) Second law of thermodynamics : Need for second law of thermodynamics, statements of second law of thermodynamics, concept of entropy, entropy as a state function of V & T,P&T, entropy change in phase change for ideal gas, entropy as criteria of spontaneity & equilibrium. [4L] (B) Free energy fun ...
uplift luna ap chemistry
... Naming is the trickiest part! Once you have been given the name, the formula writing is easy as long as you have memorized the formulas and charges of the polyatomic ions. The prefixes of a molecular compound make it really easy to write the formula since the prefix tells you how many atoms are pres ...
... Naming is the trickiest part! Once you have been given the name, the formula writing is easy as long as you have memorized the formulas and charges of the polyatomic ions. The prefixes of a molecular compound make it really easy to write the formula since the prefix tells you how many atoms are pres ...