nuclear chemistry - Wood County Schools
... protons and neutrons in the nucleus. Below is a sample nuclear reaction, the decay of uranium-238 to thorium-234: Notice that the top numbers are conserved: 238 = 4 + 234. The bottom numbers are as well: 92 = 2 + 90. Another example is the decay of carbon-14 into nitrogen-14: In this case, the botto ...
... protons and neutrons in the nucleus. Below is a sample nuclear reaction, the decay of uranium-238 to thorium-234: Notice that the top numbers are conserved: 238 = 4 + 234. The bottom numbers are as well: 92 = 2 + 90. Another example is the decay of carbon-14 into nitrogen-14: In this case, the botto ...
1 The Nucleus Total number of nucleons: mass number Number of
... inhibitors of a protein called HMGA-CoA reductase, and they help to control cholesterol biosynthesis and limit cardiovascular ...
... inhibitors of a protein called HMGA-CoA reductase, and they help to control cholesterol biosynthesis and limit cardiovascular ...
Basic properties of atomic nuclei
... When the total number of nucleons A is even, j is an integer; when it is odd, j is a half-integer. All nuclides for which both Z and N are even have 1 = 0, which suggests that pairing of particles with opposite spin components may be an important consideration in nuclear structure. nuclear magneton ...
... When the total number of nucleons A is even, j is an integer; when it is odd, j is a half-integer. All nuclides for which both Z and N are even have 1 = 0, which suggests that pairing of particles with opposite spin components may be an important consideration in nuclear structure. nuclear magneton ...
2.4 Chemical Reactions and Enzymes
... Chemical reactions that release energy often occur on their own, or spontaneously. ...
... Chemical reactions that release energy often occur on their own, or spontaneously. ...
nuclear radiation
... transmutation and became an atom of another element. The two types of radiation he found were: •The alpha particle (a) •The beta particle (b) A third type of radiation that was discovered later is called: ...
... transmutation and became an atom of another element. The two types of radiation he found were: •The alpha particle (a) •The beta particle (b) A third type of radiation that was discovered later is called: ...
Physics 228 Today: April 22, 2012 Ch. 43 Nuclear
... and binding energy make a small difference, but usually if we ignore the inclusion of electrons it is not an issue, since the number of nucleons and electrons is constant for all processes we will consider here. The total nuclear binding energy is the total energy needed to separate a nucleus into i ...
... and binding energy make a small difference, but usually if we ignore the inclusion of electrons it is not an issue, since the number of nucleons and electrons is constant for all processes we will consider here. The total nuclear binding energy is the total energy needed to separate a nucleus into i ...
Final Exam
... Physical Science Final Exam 1. Over time, there have been many scientists who have proposed different models of the atom. According to the most current atomic theory, where are the electrons located? A. in a dense, puddinglike region with the protons B. in circular orbits around the nucleus C. in th ...
... Physical Science Final Exam 1. Over time, there have been many scientists who have proposed different models of the atom. According to the most current atomic theory, where are the electrons located? A. in a dense, puddinglike region with the protons B. in circular orbits around the nucleus C. in th ...
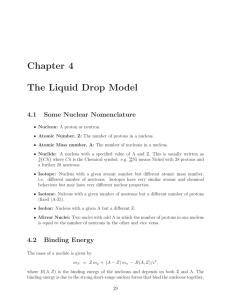
Chapter 4 The Liquid Drop Model
... other nucleons inside the nucleus, so that their binding energy is reduced. This leads to a reduction of the binding energy proportional to the surface area of the drop, i.e. proportional to A2/3 −aS A2/3 . 3. Coulomb term: Although the binding energy is mainly due to the strong nuclear force, the b ...
... other nucleons inside the nucleus, so that their binding energy is reduced. This leads to a reduction of the binding energy proportional to the surface area of the drop, i.e. proportional to A2/3 −aS A2/3 . 3. Coulomb term: Although the binding energy is mainly due to the strong nuclear force, the b ...
Energy Basics
... Nuclear Power Plant – The rate at which the nuclear fission chain reaction takes place is controlled. In conventional nuclear fission reactors, the splitting of uranium235 nuclei releases energy in form of heat, which produces high-pressure steam to spin turbines and thus generate electricity. ...
... Nuclear Power Plant – The rate at which the nuclear fission chain reaction takes place is controlled. In conventional nuclear fission reactors, the splitting of uranium235 nuclei releases energy in form of heat, which produces high-pressure steam to spin turbines and thus generate electricity. ...
Outline Chapter 8 The Nucleus 8-1. J.J. Thompson`s Plum Pudding
... has the potential of becoming the ultimate source of energy on earth. ...
... has the potential of becoming the ultimate source of energy on earth. ...
enthalpy worksheet
... Almost all chemical and physical reactions involve energy (usually in the form of heat) being released or added. An exothermic change is a reaction that releases energy. An endothermic change is one in which the energy must be added for the reaction to occur. For exothermic reactions, energy can be ...
... Almost all chemical and physical reactions involve energy (usually in the form of heat) being released or added. An exothermic change is a reaction that releases energy. An endothermic change is one in which the energy must be added for the reaction to occur. For exothermic reactions, energy can be ...
Word - The Chemistry Book
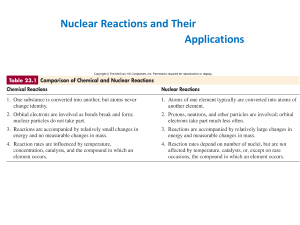
... b. First to artificially transmute one element into another B. Nuclear reactions 1. Involve great quantities of energy Strong nuclear force overcomes electrostatic repulsion between protons 2. Differ from chemical reactions a. atomic numbers change b. some matter is changed to energy c. specific iso ...
... b. First to artificially transmute one element into another B. Nuclear reactions 1. Involve great quantities of energy Strong nuclear force overcomes electrostatic repulsion between protons 2. Differ from chemical reactions a. atomic numbers change b. some matter is changed to energy c. specific iso ...
Chapter 29: Nuclear Physics
... The absorbed dose of ionizing radiation is the amount of radiation energy absorbed per unit mass of tissue. Ionizing radiation is radiation with enough energy to ionize an atom or molecule. The SI unit of absorbed dose is the Gray. 1 Gy = 1 J/kg. Another common unit is the rad (radiation absorbed do ...
... The absorbed dose of ionizing radiation is the amount of radiation energy absorbed per unit mass of tissue. Ionizing radiation is radiation with enough energy to ionize an atom or molecule. The SI unit of absorbed dose is the Gray. 1 Gy = 1 J/kg. Another common unit is the rad (radiation absorbed do ...
7.3 – Nuclear Reactions, Fission and Fusion 7.3.1 – Describe and
... Students must be familiar with the units MeV c -2 and GeV c -2 for mass 7.3.4 - Apply the Einstein mass – energy equivalence relationship 7.3.5 – Define the concepts of mass defect, binding energy and binding energy per nucleon 7.3.6 – Draw and annotate a graph showing the variation with nucleon num ...
... Students must be familiar with the units MeV c -2 and GeV c -2 for mass 7.3.4 - Apply the Einstein mass – energy equivalence relationship 7.3.5 – Define the concepts of mass defect, binding energy and binding energy per nucleon 7.3.6 – Draw and annotate a graph showing the variation with nucleon num ...
Chapter 30: Nuclear Physics What will we learn in this chapter?
... When Q > 0, the total mass decreases and we have an exoergic reaction; the kinetic energy increases. When Q < 0, the total mass increases and we have an endoergic reaction; the kinetic energy decreases. An endoergic reaction cannot occur unless the initial kinetic energy is at least as great as |Q|; ...
... When Q > 0, the total mass decreases and we have an exoergic reaction; the kinetic energy increases. When Q < 0, the total mass increases and we have an endoergic reaction; the kinetic energy decreases. An endoergic reaction cannot occur unless the initial kinetic energy is at least as great as |Q|; ...
File
... Mass is created and energy is released. Mass is created and energy is stored. Mass is converted to energy, which is released. Mass is converted to energy, which is stored. ...
... Mass is created and energy is released. Mass is created and energy is stored. Mass is converted to energy, which is released. Mass is converted to energy, which is stored. ...
Santee Education Complex Chemistry Mini Assessment 11
... d. 7N14 + 2He4 →1H1 + 8O17 14) A process in which a very heavy nucleus splits into more stable nuclei of intermediate mass is called: a. nuclear fission. b. a chain reaction. c. nuclear fusion. d. radiocarbon dating. 15) An electron emitted from the nucleus during some kinds of radioactive decay is ...
... d. 7N14 + 2He4 →1H1 + 8O17 14) A process in which a very heavy nucleus splits into more stable nuclei of intermediate mass is called: a. nuclear fission. b. a chain reaction. c. nuclear fusion. d. radiocarbon dating. 15) An electron emitted from the nucleus during some kinds of radioactive decay is ...
File
... After completing the equation by writing all the nuclear particles in an atomic notation, a coefficient may be necessary to balance the reaction. Use a particle or isotope to fill in the missing protons and neutrons. ...
... After completing the equation by writing all the nuclear particles in an atomic notation, a coefficient may be necessary to balance the reaction. Use a particle or isotope to fill in the missing protons and neutrons. ...
Notes - Science With Horne
... After completing the equation by writing all the nuclear particles in an atomic notation, a coefficient may be necessary to balance the reaction. Use a particle or isotope to fill in the missing protons and neutrons. ...
... After completing the equation by writing all the nuclear particles in an atomic notation, a coefficient may be necessary to balance the reaction. Use a particle or isotope to fill in the missing protons and neutrons. ...
Chapter 3
... Nuclear Power Plant – The rate at which the nuclear fission chain reaction takes place is controlled. In conventional nuclear fission reactors, the splitting of uranium235 nuclei releases energy in form of heat, which produces high-pressure steam to spin turbines and thus generate electricity. ...
... Nuclear Power Plant – The rate at which the nuclear fission chain reaction takes place is controlled. In conventional nuclear fission reactors, the splitting of uranium235 nuclei releases energy in form of heat, which produces high-pressure steam to spin turbines and thus generate electricity. ...
APES-Chapter-3
... Nuclear Power Plant – The rate at which the nuclear fission chain reaction takes place is controlled. In conventional nuclear fission reactors, the splitting of uranium235 nuclei releases energy in form of heat, which produces high-pressure steam to spin turbines and thus generate electricity. ...
... Nuclear Power Plant – The rate at which the nuclear fission chain reaction takes place is controlled. In conventional nuclear fission reactors, the splitting of uranium235 nuclei releases energy in form of heat, which produces high-pressure steam to spin turbines and thus generate electricity. ...
Nuclear fusion

In nuclear physics, nuclear fusion is a nuclear reaction in which two or more atomic nuclei come very close and then collide at a very high speed and join to form a new nucleus. During this process, matter is not conserved because some of the matter of the fusing nuclei is converted to photons (energy). Fusion is the process that powers active or ""main sequence"" stars.The fusion of two nuclei with lower masses than Iron-56 (which, along with Nickel-62, has the largest binding energy per nucleon) generally releases energy, while the fusion of nuclei heavier than iron absorbs energy. The opposite is true for the reverse process, nuclear fission. This means that fusion generally occurs for lighter elements only, and likewise, that fission normally occurs only for heavier elements. There are extreme astrophysical events that can lead to short periods of fusion with heavier nuclei. This is the process that gives rise to nucleosynthesis, the creation of the heavy elements during events such as supernova.Following the discovery of quantum tunneling by Friedrich Hund, in 1929 Robert Atkinson and Fritz Houtermans used the measured masses of light elements to predict that large amounts of energy could be released by fusing small nuclei. Building upon the nuclear transmutation experiments by Ernest Rutherford, carried out several years earlier, the laboratory fusion of hydrogen isotopes was first accomplished by Mark Oliphant in 1932. During the remainder of that decade the steps of the main cycle of nuclear fusion in stars were worked out by Hans Bethe. Research into fusion for military purposes began in the early 1940s as part of the Manhattan Project. Fusion was accomplished in 1951 with the Greenhouse Item nuclear test. Nuclear fusion on a large scale in an explosion was first carried out on November 1, 1952, in the Ivy Mike hydrogen bomb test.Research into developing controlled thermonuclear fusion for civil purposes also began in earnest in the 1950s, and it continues to this day. The present article is about the theory of fusion. For details of the quest for controlled fusion and its history, see the article Fusion power.