Synthesis of biopolymers: proteins, polyesters
... characteristics of organisms.In addition to the very specific relations between structure and function, another aspect of biopolymers intriguing to materials scientists is the precision with which they are made. In contrast to conventional synthetic polymers, which show a distribution of monomer seq ...
... characteristics of organisms.In addition to the very specific relations between structure and function, another aspect of biopolymers intriguing to materials scientists is the precision with which they are made. In contrast to conventional synthetic polymers, which show a distribution of monomer seq ...
Lecture 1
... • 1850’s: First amino acids isolated from natural products • 1903-1906: By hydrolysis of natural proteins, Emil Fischer proves that they are copolymers of amino acids (strange, but none of his so fundamental papers earned more than ~60 citations!). • 1930’s and 1940’s: proteins are viewed as spheroi ...
... • 1850’s: First amino acids isolated from natural products • 1903-1906: By hydrolysis of natural proteins, Emil Fischer proves that they are copolymers of amino acids (strange, but none of his so fundamental papers earned more than ~60 citations!). • 1930’s and 1940’s: proteins are viewed as spheroi ...
Structural Biochemistry/Metabolism
... a. phospholipids consist of a glycerol, two fatty acid and a phosphate group attaching to an alcohol group b. phospholipids consist of hydrophilic head and hydrophobic tail c. phospholipids made up the cell membranes' bilayer 1) hydrophobic tails dislike water and try to get away by getting as close ...
... a. phospholipids consist of a glycerol, two fatty acid and a phosphate group attaching to an alcohol group b. phospholipids consist of hydrophilic head and hydrophobic tail c. phospholipids made up the cell membranes' bilayer 1) hydrophobic tails dislike water and try to get away by getting as close ...
Max ARM (Anabolic Recovery Matrix) from Max Muscle Sports
... stimulation of p70 ribosomal S6 kinase (p70 S6K) through enhanced translation of specific mRNAs. The Akt/mTOR pathway in muscle is upregulated during the hypertrophy (increase in muscle size) phase.† An exciting area in the molecular biochemistry of protein synthesis are the role of Heat Shock Prote ...
... stimulation of p70 ribosomal S6 kinase (p70 S6K) through enhanced translation of specific mRNAs. The Akt/mTOR pathway in muscle is upregulated during the hypertrophy (increase in muscle size) phase.† An exciting area in the molecular biochemistry of protein synthesis are the role of Heat Shock Prote ...
KTH | BB2160 Structure Biology 7.5 credits
... structure is determined, most importantly using the method of X-ray crystallography. During the seminar project, you will study, in detail, a specific protein structure and its function. Guided by information retrieved from the course and scientific articles, you should be able to analyze, validate ...
... structure is determined, most importantly using the method of X-ray crystallography. During the seminar project, you will study, in detail, a specific protein structure and its function. Guided by information retrieved from the course and scientific articles, you should be able to analyze, validate ...
Document
... •Receptors These proteins are responsible for signal detection and translation into other type of signal. •Signalling proteins - This group of proteins is involved into signaling transduction process. •Storage proteins. These proteins contain energy, which can be released during metabolism processes ...
... •Receptors These proteins are responsible for signal detection and translation into other type of signal. •Signalling proteins - This group of proteins is involved into signaling transduction process. •Storage proteins. These proteins contain energy, which can be released during metabolism processes ...
answers to "extra stuff" practice problems
... the C terminus, and the carboxyl group which is part of the side chain of aspartic acid.) The highest pKa (8.60) thus gets assigned to the N terminus. To distinguish between the other two, the carboxyl group closer to the electronwithdrawing NH3+ is the one that is more acidic, with the lower pKa. T ...
... the C terminus, and the carboxyl group which is part of the side chain of aspartic acid.) The highest pKa (8.60) thus gets assigned to the N terminus. To distinguish between the other two, the carboxyl group closer to the electronwithdrawing NH3+ is the one that is more acidic, with the lower pKa. T ...
Valea LifeScience09 R
... via its amino acid sequence, however, leads to the delicate problem of how to present this sequence to obtain patent protection that actually corresponds to the invention’s contribution to the art (in line with the teachings of T409/91 and T435/91). For instance, the amino acid sequences of many nat ...
... via its amino acid sequence, however, leads to the delicate problem of how to present this sequence to obtain patent protection that actually corresponds to the invention’s contribution to the art (in line with the teachings of T409/91 and T435/91). For instance, the amino acid sequences of many nat ...
Name____________________________________________
... The movement of a particle up a concentration gradient helped by active pumping. ...
... The movement of a particle up a concentration gradient helped by active pumping. ...
View video content as a PDF
... An alpha helix can be folded by wrapping the toober around a finger. In the zinc finger sample protein, there is an alpha helix from amino acid 19 to amino acid 30. This entire area should be folded into an alpha helix. It is important to make extra sure that your alpha helices are always right hand ...
... An alpha helix can be folded by wrapping the toober around a finger. In the zinc finger sample protein, there is an alpha helix from amino acid 19 to amino acid 30. This entire area should be folded into an alpha helix. It is important to make extra sure that your alpha helices are always right hand ...
As Powerpoint Slide
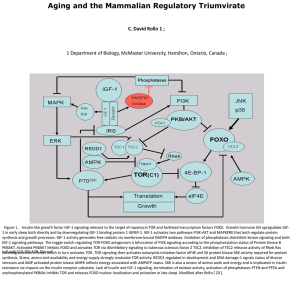
... 1 Department of Biology, McMaster University, Hamilton, Ontario, Canada ; ...
... 1 Department of Biology, McMaster University, Hamilton, Ontario, Canada ; ...
Student Exploration Sheet: Growing Plants
... asparagine, valine, and histidine. Give an mRNA sequence that would code for this protein. ...
... asparagine, valine, and histidine. Give an mRNA sequence that would code for this protein. ...
Organic Molecules Worksheet:
... Organic molecules have four common characteristics. First, they are all carbon based, meaning they all contain carbon. They are formed from just a few elements that join together to form small molecules that join together, or bond, to form large molecules. The third characteristic of all organic mol ...
... Organic molecules have four common characteristics. First, they are all carbon based, meaning they all contain carbon. They are formed from just a few elements that join together to form small molecules that join together, or bond, to form large molecules. The third characteristic of all organic mol ...
Student Exploration Sheet: Growing Plants
... asparagine, valine, and histidine. Give an mRNA sequence that would code for this protein. ...
... asparagine, valine, and histidine. Give an mRNA sequence that would code for this protein. ...
New degradation proteins show route to cell survival
... autophagy of a certain cell constituent – the endoplasmic reticulum (ER), a network of flattened membrane enclosed sacks – in nitrogen-starved conditions. The same conditions also triggered degradation of a part of the nucleus by Atg39; this protein localized to a special part of the ER surrounding ...
... autophagy of a certain cell constituent – the endoplasmic reticulum (ER), a network of flattened membrane enclosed sacks – in nitrogen-starved conditions. The same conditions also triggered degradation of a part of the nucleus by Atg39; this protein localized to a special part of the ER surrounding ...
Page 1 - csfcbiology
... The skin is one of the largest organs in the body. It is composed of several layers of tissue. The outer layer consists of dead cells packed with keratins. Keratins are a group of proteins that differ from each other in their primary structure. Each keratin molecule consists of several polypeptide c ...
... The skin is one of the largest organs in the body. It is composed of several layers of tissue. The outer layer consists of dead cells packed with keratins. Keratins are a group of proteins that differ from each other in their primary structure. Each keratin molecule consists of several polypeptide c ...
Chap. 5 Video Notes Outline
... The phospholipid bilayer is fluid. What does this mean and why is it important to the cell? _________________ _________________________________________________________________________________________________________________________ ____________________________________________________________________ ...
... The phospholipid bilayer is fluid. What does this mean and why is it important to the cell? _________________ _________________________________________________________________________________________________________________________ ____________________________________________________________________ ...
MCB Lecture 3 – ER and Golgi
... Once the Polypeptide Chain is shuttled into the ER through the translocon, what protein helps it fold correctly? o BiP – Binding Protein What enzyme in the ER Lumen cleaves the N-Terminal Signal from the Polypeptide chain as it enters the lumen? o Signal Peptidase What is needed for a Single-Pass Tr ...
... Once the Polypeptide Chain is shuttled into the ER through the translocon, what protein helps it fold correctly? o BiP – Binding Protein What enzyme in the ER Lumen cleaves the N-Terminal Signal from the Polypeptide chain as it enters the lumen? o Signal Peptidase What is needed for a Single-Pass Tr ...
Protein Ubiquitination
... Chaperons: Are a group of proteins playing important role in protein folding. Chaperons safeguard the folding of nascent chains. ...
... Chaperons: Are a group of proteins playing important role in protein folding. Chaperons safeguard the folding of nascent chains. ...
Lecture20_Translation
... Post-translational modifications are required by some proteins • Some proteins require modification before the fully active conformation is achieved • Post-translational modifications include: – Enzymatic removal of formyl group from first residue, or removal of Met and sometimes additional residue ...
... Post-translational modifications are required by some proteins • Some proteins require modification before the fully active conformation is achieved • Post-translational modifications include: – Enzymatic removal of formyl group from first residue, or removal of Met and sometimes additional residue ...
nucleotides and nucleic acids
... d. 100 * e. 1,000 44.A solution with a pH of 8 has how many times fewer hydrogen ions than a solution with a pH of 6? a. 2 b. 4 c. 10 * d. 100 e. 1,000 45.The three most common atoms in your body are * a. hydrogen, oxygen, and carbon. b. carbon, hydrogen, and nitrogen. c. carbon, nitrogen, and oxyge ...
... d. 100 * e. 1,000 44.A solution with a pH of 8 has how many times fewer hydrogen ions than a solution with a pH of 6? a. 2 b. 4 c. 10 * d. 100 e. 1,000 45.The three most common atoms in your body are * a. hydrogen, oxygen, and carbon. b. carbon, hydrogen, and nitrogen. c. carbon, nitrogen, and oxyge ...
File - Ms. Poole`s Biology
... • mRNA-carries the information from the DNA gene to the cytoplasm. Determines the sequence of amino acids for a protein • tRNA-brings the correct amino acid to the ribosome and mRNA in translation • rRNA-found on ribosomes and used to "connect" the tRNA to the ...
... • mRNA-carries the information from the DNA gene to the cytoplasm. Determines the sequence of amino acids for a protein • tRNA-brings the correct amino acid to the ribosome and mRNA in translation • rRNA-found on ribosomes and used to "connect" the tRNA to the ...
Electrophoresis of Serum Proteins Properties of Proteins
... Solubility of a protein in water is basically determined by the presence of polar amino acid residues in its primary structure. Some proteins dissolve easily in water (e.g. albumin), others not at all (e.g. collagen). Speaking only about proteins that are basically water soluble, stability of aqueou ...
... Solubility of a protein in water is basically determined by the presence of polar amino acid residues in its primary structure. Some proteins dissolve easily in water (e.g. albumin), others not at all (e.g. collagen). Speaking only about proteins that are basically water soluble, stability of aqueou ...
The Structure of Proteins
... such as diketopiperazine which are known to con- heat of solution of amides and alcohols show that tain polypeptide chains or rings. It is possible, the stability relations in solution are little differhowever, to make this comparison indirectly in ent from those of the crystalline substances. We va ...
... such as diketopiperazine which are known to con- heat of solution of amides and alcohols show that tain polypeptide chains or rings. It is possible, the stability relations in solution are little differhowever, to make this comparison indirectly in ent from those of the crystalline substances. We va ...
Chapter 12. Protein biosynthesis (P215, sP875)
... biosynthesis, which is read in a 5’3’ direction. Each three nucleotides form a codon representing for a specific amino acid. Thus, the base sequence of an mRNA molecule determines the amino acid sequence of the protein. ...
... biosynthesis, which is read in a 5’3’ direction. Each three nucleotides form a codon representing for a specific amino acid. Thus, the base sequence of an mRNA molecule determines the amino acid sequence of the protein. ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.