Dr Asmat Salim MM707-electrophoresis 2014
... and will move if electric field is applied. In electrophoresis, macromolecules are characterized by their rate of movement in an electric field. This technique is used to (1) distinguish molecules on the basis of charge and shape (2) to determine molecular weight of proteins (3) to detect amino a ...
... and will move if electric field is applied. In electrophoresis, macromolecules are characterized by their rate of movement in an electric field. This technique is used to (1) distinguish molecules on the basis of charge and shape (2) to determine molecular weight of proteins (3) to detect amino a ...
The Ubiquitin Proteosome pathway
... Several complex processes are mediated via degradation or processing of specific proteins. Aberrations in these systems associates with pathogenic ...
... Several complex processes are mediated via degradation or processing of specific proteins. Aberrations in these systems associates with pathogenic ...
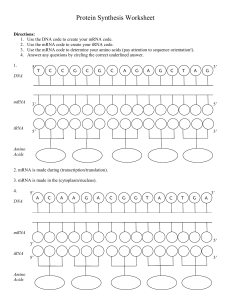
Protein Synthesis Worksheet
... 23. (large ribosomal subunit/MET tRNA) Second item to bind to the developing translation complex (after ...
... 23. (large ribosomal subunit/MET tRNA) Second item to bind to the developing translation complex (after ...
Exploring Proteins - Weber State University
... • Immunoglobulins (antibodies) can be utilized as powerful analytical tools in biochemistry • An antibody (Immunoglobulin, Ig) is a complex protein formed by an animal in response to the presence of a foreign substance (most often foreign proteins). • An antibody usually exhibits specific and high ...
... • Immunoglobulins (antibodies) can be utilized as powerful analytical tools in biochemistry • An antibody (Immunoglobulin, Ig) is a complex protein formed by an animal in response to the presence of a foreign substance (most often foreign proteins). • An antibody usually exhibits specific and high ...
Lecture notes Chapter 22-23
... polypeptide subunits, the structure level is referred to as a quaternary structure. Hemoglobin, a globular protein that transports oxygen in blood, consists of four polypeptide chains or subunits, two α chains, and two chains. The subunits are held together in the quaternary by the same interactio ...
... polypeptide subunits, the structure level is referred to as a quaternary structure. Hemoglobin, a globular protein that transports oxygen in blood, consists of four polypeptide chains or subunits, two α chains, and two chains. The subunits are held together in the quaternary by the same interactio ...
91.510_ch8_part1
... Definition of a motif A motif (or fingerprint) is a short, conserved region of a protein. Its size is often 10 to 20 amino acids. Simple motifs include transmembrane domains and phosphorylation sites. These do not imply homology when found in a group of proteins. PROSITE (www.expasy.org/prosite) is ...
... Definition of a motif A motif (or fingerprint) is a short, conserved region of a protein. Its size is often 10 to 20 amino acids. Simple motifs include transmembrane domains and phosphorylation sites. These do not imply homology when found in a group of proteins. PROSITE (www.expasy.org/prosite) is ...
Biological Molecules: Water and Carbohydrates
... main biological molecules it is appropriate to gain an understanding of the cell membrane before going on to the last important biochemical molecule. ...
... main biological molecules it is appropriate to gain an understanding of the cell membrane before going on to the last important biochemical molecule. ...
Document
... • tRNA molecules bring amino acids to the mRNA. • Peptide bonds form between an amino acid and the end of the ...
... • tRNA molecules bring amino acids to the mRNA. • Peptide bonds form between an amino acid and the end of the ...
ppt file
... acceptors be hydrogen bonded to something, be it solvent, protein backbone, or protein side chains. Alan Fersht has called this concept “hydrogen bond inventory”. This is important when trying to understand the effect of mutations that impact hydrogen bonding, because removal of one partner of a hyd ...
... acceptors be hydrogen bonded to something, be it solvent, protein backbone, or protein side chains. Alan Fersht has called this concept “hydrogen bond inventory”. This is important when trying to understand the effect of mutations that impact hydrogen bonding, because removal of one partner of a hyd ...
Biochemistry Test Review
... 35. What are several types of carbohydrate polymers and what are their roles in living things? 36. What is an alternative name for "starch" that identifies it as a type of poly-sugar (based on the ...
... 35. What are several types of carbohydrate polymers and what are their roles in living things? 36. What is an alternative name for "starch" that identifies it as a type of poly-sugar (based on the ...
Advances in Amino Acid Analysis
... Ala, Leu, Lys, and Gly — are well recovered and commonly used for protein quantitation according to their residue yields. Actually, eight residues are well recovered because Asn and Gln are converted to Asp and Glu by deamidation under harsh acid conditions. ...
... Ala, Leu, Lys, and Gly — are well recovered and commonly used for protein quantitation according to their residue yields. Actually, eight residues are well recovered because Asn and Gln are converted to Asp and Glu by deamidation under harsh acid conditions. ...
VCE Biology FAQs
... cholesterol is not required. Students are expected to understand that polypeptides and proteins are polymers of amino acids, formed through condensation reactions. They are also expected to understand that the primary structure of a polypeptide or protein is the sequence of amino acids that form the ...
... cholesterol is not required. Students are expected to understand that polypeptides and proteins are polymers of amino acids, formed through condensation reactions. They are also expected to understand that the primary structure of a polypeptide or protein is the sequence of amino acids that form the ...
Amino-Form
... When ammonium and nitrates are absorbed by roots or leaves, they are first converted into Glutamic acid, one of the most important Lform amino acids. All the other amino acids are then formed by the reaction of organic molecules with Glutamic acid. A percentage of these amino acids will combine to f ...
... When ammonium and nitrates are absorbed by roots or leaves, they are first converted into Glutamic acid, one of the most important Lform amino acids. All the other amino acids are then formed by the reaction of organic molecules with Glutamic acid. A percentage of these amino acids will combine to f ...
Model Description Sheet
... conditions is hypersensitivity to touch, where daily activities can be painful. Few therapeutics to ameliorate mechanical hypersensitivity exist because the mammalian ion channels that sense touch are poorly understood. The mechanosensitive channel of large conductance (MscL) is an ion channel in My ...
... conditions is hypersensitivity to touch, where daily activities can be painful. Few therapeutics to ameliorate mechanical hypersensitivity exist because the mammalian ion channels that sense touch are poorly understood. The mechanosensitive channel of large conductance (MscL) is an ion channel in My ...
Amino Acids : BCAA FLASH ZERO 360GR - BIOTECH
... Amino acids are the building blocks of one of our fundamental nutrients, proteins, commonly found everywhere in human body. There are some amino acids which human body is capable of producing (non-essential amino acids), whereas the amino acids belonging to the other group (essential amino acids) ar ...
... Amino acids are the building blocks of one of our fundamental nutrients, proteins, commonly found everywhere in human body. There are some amino acids which human body is capable of producing (non-essential amino acids), whereas the amino acids belonging to the other group (essential amino acids) ar ...
Active and passive mechanisms of intracellular transport and
... nelles, and the control of cell polarity [1–4]. Recently developed high-resolution fluorescence techniques revealed that many proteins are sorted to specific locations to achieve their functions. These include not only receptors and signaling proteins but also proteases and other metabolic enzymes. ...
... nelles, and the control of cell polarity [1–4]. Recently developed high-resolution fluorescence techniques revealed that many proteins are sorted to specific locations to achieve their functions. These include not only receptors and signaling proteins but also proteases and other metabolic enzymes. ...
RBT1, a novel transcriptional co-activator, binds the second subunit
... derived from cell line MCF-7 and cloned into the yeast twohybrid plasmids pBTM116 and pACT2 in frame to LexA (1–202) and GAL4-TA, respectively. Similarly, both XPA and UDG nucleotide coding sequences were PCR amplified from cDNA derived from cell line normal non-immortalized human mammary epithelial ...
... derived from cell line MCF-7 and cloned into the yeast twohybrid plasmids pBTM116 and pACT2 in frame to LexA (1–202) and GAL4-TA, respectively. Similarly, both XPA and UDG nucleotide coding sequences were PCR amplified from cDNA derived from cell line normal non-immortalized human mammary epithelial ...
Introduction- Amino acid protection and deprotection is particularly
... intermediate in organic synthesis there is variety of reagent for conversion of amino acid to amino acid ester (2). Amino acid protection and deprotection is also used in peptide synthesis of amino acid in solid and solution phase synthesis , the advantage of solution phase synthesis is to isolate a ...
... intermediate in organic synthesis there is variety of reagent for conversion of amino acid to amino acid ester (2). Amino acid protection and deprotection is also used in peptide synthesis of amino acid in solid and solution phase synthesis , the advantage of solution phase synthesis is to isolate a ...
Protein Structure and Function
... -Citrate synthase with a different geometry from that of the substrate -Asp375 and His274 catalyze the formation of the enol of acetyl-CoA -The acetyl-CoA enol attacks the carbonyl carbon of oxaloacetate -Addition of the elements of the acetyl group at this portion Figure2-28.The active site of citr ...
... -Citrate synthase with a different geometry from that of the substrate -Asp375 and His274 catalyze the formation of the enol of acetyl-CoA -The acetyl-CoA enol attacks the carbonyl carbon of oxaloacetate -Addition of the elements of the acetyl group at this portion Figure2-28.The active site of citr ...
carbon skeleton
... Some amino acids that are released from protein breakdown and are not needed for new protein synthesis undergo oxidative degradation. When a diet is rich in protein and the ingested amino acids exceed the body’s needs for protein synthesis, the surplus is catabolized; amino acids cannot be stored. D ...
... Some amino acids that are released from protein breakdown and are not needed for new protein synthesis undergo oxidative degradation. When a diet is rich in protein and the ingested amino acids exceed the body’s needs for protein synthesis, the surplus is catabolized; amino acids cannot be stored. D ...
Lecture 17 Outline Cell Motility: Encompasses both changes in cell
... movement of cilia different but mechanism same. Key is axonemal dynein that can bind MT at head and tail. Cross bridges between the neighboring tubule pairs ( via Nexin protein) allows movement of ciliary dyneins to not cause sliding of one filament over other, instead, bending of cilia or flagella. ...
... movement of cilia different but mechanism same. Key is axonemal dynein that can bind MT at head and tail. Cross bridges between the neighboring tubule pairs ( via Nexin protein) allows movement of ciliary dyneins to not cause sliding of one filament over other, instead, bending of cilia or flagella. ...
H - Free
... Functional groups on biomolecule surface: proteins Enzymes, antibodies (and of course receptor proteins) are all proteins A number of their amino acid building blocks have functional side chains that can be used for chemical attachment Amino acids with functional groups: Lysine (NH2), Cysteine (SH) ...
... Functional groups on biomolecule surface: proteins Enzymes, antibodies (and of course receptor proteins) are all proteins A number of their amino acid building blocks have functional side chains that can be used for chemical attachment Amino acids with functional groups: Lysine (NH2), Cysteine (SH) ...
Monomeric state and ligand binding of recombinant GABA transporter Xiao-Dan Li
... planar heterocyclic compounds without a carboxyl group [5]. Some of these inhibitors are also substrates for they can be translocated across the membrane by GabP under appropriate conditions [6]. E. coli GABA transporter is a member of the amino acid/ polyamine/organocation (APC) transporter superfa ...
... planar heterocyclic compounds without a carboxyl group [5]. Some of these inhibitors are also substrates for they can be translocated across the membrane by GabP under appropriate conditions [6]. E. coli GABA transporter is a member of the amino acid/ polyamine/organocation (APC) transporter superfa ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.