CHMI 2227E Biochemistry I
... Positively charged proteins migrate towards the negative cathode; Negatively charged proteins migrate towards the positive anode; ...
... Positively charged proteins migrate towards the negative cathode; Negatively charged proteins migrate towards the positive anode; ...
Organization of the Kidney Proximal
... ability to attack peptides such as insulin B chain and glucagon, but not more highly structured proteins such as insulin, albumin or casein. The specificity shown by the endopeptidase is similar to that of the group of microbial neutral proteinases typified by thermolysin :the peptide bonds attacked ...
... ability to attack peptides such as insulin B chain and glucagon, but not more highly structured proteins such as insulin, albumin or casein. The specificity shown by the endopeptidase is similar to that of the group of microbial neutral proteinases typified by thermolysin :the peptide bonds attacked ...
pdf-version
... "no membrane" will completely hide the membranes, which is useful for nonmembrane proteins. "automatic" will come up with a transmembrane topology depending on your input sequence: for UniProt identifiers it will use the annotated topology, and for amino acid sequences it will use a transmembrane to ...
... "no membrane" will completely hide the membranes, which is useful for nonmembrane proteins. "automatic" will come up with a transmembrane topology depending on your input sequence: for UniProt identifiers it will use the annotated topology, and for amino acid sequences it will use a transmembrane to ...
N - KIAS
... • Mechanisms of Structural organization • Nature of the Folding Nuclei • Interactions that guide folding (native vs nonnative) • Folding rates – dependence on N ...
... • Mechanisms of Structural organization • Nature of the Folding Nuclei • Interactions that guide folding (native vs nonnative) • Folding rates – dependence on N ...
Transcription
... Fine structure of the gene Cistron - basic unit of function , which determines the sequence of amino acids in a particular protein. Cistron - is synonymous with gene. Recon is an elementary unit of recombination in crossing over . It is a pair of nucleotides. Mouton basic unit of genetic variabilit ...
... Fine structure of the gene Cistron - basic unit of function , which determines the sequence of amino acids in a particular protein. Cistron - is synonymous with gene. Recon is an elementary unit of recombination in crossing over . It is a pair of nucleotides. Mouton basic unit of genetic variabilit ...
Michael S. Chimenti PhD “Michael has been the linchpin in our
... Predicted a novel hyper-phosphorylated regulatory mechanism in CLASP2 using explicit solvent molecular dynamics and other computational tools Demonstrated the correctness of computational predictions by designing and carrying out fluorescence polarization assays of wtCLASP2 and mutants to measure af ...
... Predicted a novel hyper-phosphorylated regulatory mechanism in CLASP2 using explicit solvent molecular dynamics and other computational tools Demonstrated the correctness of computational predictions by designing and carrying out fluorescence polarization assays of wtCLASP2 and mutants to measure af ...
Model Description Sheet
... along with tetanus toxin, are produced by several species of Clostridium. All clostridial neurotoxins, such as BoNT/A, are di-chain proteins, consisting of a 50-kDa light chain, the catalytic domain, connected by a disulfide bond to a 100-kDa heavy chain, containing the receptor-binding and transloc ...
... along with tetanus toxin, are produced by several species of Clostridium. All clostridial neurotoxins, such as BoNT/A, are di-chain proteins, consisting of a 50-kDa light chain, the catalytic domain, connected by a disulfide bond to a 100-kDa heavy chain, containing the receptor-binding and transloc ...
Amino acids and peptide bonds
... All amino acids can be based on one of three basic groups, non-polar, uncharged polar and charged polar (table 4-1). Also are classified based on hydrophobicity, reactivity, acidic and basic nature, relative size of the side groups ...
... All amino acids can be based on one of three basic groups, non-polar, uncharged polar and charged polar (table 4-1). Also are classified based on hydrophobicity, reactivity, acidic and basic nature, relative size of the side groups ...
Proteomics of Poxvirus - KEIVAN BEHESHTI MAAL'S HOMEPAGE
... most essential genes located in the central part of the genome (highly consereved) e.g assembly and replication ...
... most essential genes located in the central part of the genome (highly consereved) e.g assembly and replication ...
Biomolecules
... Made of C,H,O,N Functions: Build body structures, control chemical reactions, do cellular work Example: meat/muscle, hair, nails, enzymes, peanut butter, milk Monomer: amino acid ...
... Made of C,H,O,N Functions: Build body structures, control chemical reactions, do cellular work Example: meat/muscle, hair, nails, enzymes, peanut butter, milk Monomer: amino acid ...
concentration gradient
... COOPERATIVE STRUCTURES 1. They are held together by many reinforcing non-covalent interactions, which makes them extensive. 2. They close on themselves so there are no edges with hydrocarbon chains exposed to water, which favors compartmentalization. 3. They are self-sealing because a hole is energe ...
... COOPERATIVE STRUCTURES 1. They are held together by many reinforcing non-covalent interactions, which makes them extensive. 2. They close on themselves so there are no edges with hydrocarbon chains exposed to water, which favors compartmentalization. 3. They are self-sealing because a hole is energe ...
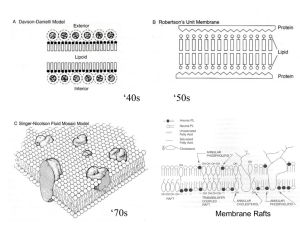
lecture 11
... 4 reviews on domain formation in model membranes and physical properties that underlie raft formation 2 reviews to describe techniques used for studying rafts (FRET) – and uncertainty for detecting rafts in cell membranes Raft Function in Cells: 4 on signal transduction(IgE receptor signaling, Growt ...
... 4 reviews on domain formation in model membranes and physical properties that underlie raft formation 2 reviews to describe techniques used for studying rafts (FRET) – and uncertainty for detecting rafts in cell membranes Raft Function in Cells: 4 on signal transduction(IgE receptor signaling, Growt ...
Nucleic Acids
... – Perform most of the tasks the body needs to function – Form enzymes, chemicals that change the rate of a chemical reaction without being changed in the process ...
... – Perform most of the tasks the body needs to function – Form enzymes, chemicals that change the rate of a chemical reaction without being changed in the process ...
Amino acids
... – His has a side chain pKa of 6.0 and is only 10% protonated at pH 7 – Because His has a pKa near neutral, it plays important roles as a proton donor or acceptor in many enzymes. – His containing peptides are important biological buffers ...
... – His has a side chain pKa of 6.0 and is only 10% protonated at pH 7 – Because His has a pKa near neutral, it plays important roles as a proton donor or acceptor in many enzymes. – His containing peptides are important biological buffers ...
Non-Essential Amino Acids
... of one amino acid and the amino group of the next amino acid. These bonds are formed using dehydration synthesis. ...
... of one amino acid and the amino group of the next amino acid. These bonds are formed using dehydration synthesis. ...
Molecule-Metabolism ppt
... diversity of stable compounds to exist. Despite only being the 15th most abundant element on the planet carbon forms the backbone of every single organic molecule. Covalent bonds are the strongest type of bond between atoms. Stable molecules can be formed. ...
... diversity of stable compounds to exist. Despite only being the 15th most abundant element on the planet carbon forms the backbone of every single organic molecule. Covalent bonds are the strongest type of bond between atoms. Stable molecules can be formed. ...
Chapter 5 Membrane Structure and Function
... – Polar heads face _______ _______________ – Non-polar tails mingle _______ the membrane – Cholesterol in animal membranes keeps them ___________ ...
... – Polar heads face _______ _______________ – Non-polar tails mingle _______ the membrane – Cholesterol in animal membranes keeps them ___________ ...
Help Wanted
... workers. No previous experience necessary. Must be able to transcribe code in a nuclear environment. The ability to work in close association with ribosomes is a must. Accuracy and Speed vital for this job in the field of translation. Applicants must demonstrate skills in transporting and positionin ...
... workers. No previous experience necessary. Must be able to transcribe code in a nuclear environment. The ability to work in close association with ribosomes is a must. Accuracy and Speed vital for this job in the field of translation. Applicants must demonstrate skills in transporting and positionin ...
What is bioinformatics? A proposed definition and overview of the field
... genes, which at its most basic can be viewed as digital information. At the same time, there have been major advances in the technologies that supply the initial data; Anthony Kervalage of Celera recently cited that an experimental laboratory can produce over 100 gigabytes of data a day with ease (5 ...
... genes, which at its most basic can be viewed as digital information. At the same time, there have been major advances in the technologies that supply the initial data; Anthony Kervalage of Celera recently cited that an experimental laboratory can produce over 100 gigabytes of data a day with ease (5 ...
PHYSIOLOGY LECTURE EXAM #1 REVIEW LIST
... -what is chromatin? How is it organized? -what is the nucleolus? What does it do? -what is the nucleoplasm? -what is the nuclear envelope? -how do materials enter/exit the nucleus? -know the base-pairing rules of DNA -how do the bases join together in a DNA strand? -how are the two strand of DNA ori ...
... -what is chromatin? How is it organized? -what is the nucleolus? What does it do? -what is the nucleoplasm? -what is the nuclear envelope? -how do materials enter/exit the nucleus? -know the base-pairing rules of DNA -how do the bases join together in a DNA strand? -how are the two strand of DNA ori ...
Sequence Motif Identification and Protein Family - IME-USP
... protein using the information contained in its amino acid sequence [1]. Nowadays, the most popular methods to generate a hypothesis about the function of a protein are BLAST and Hidden Markov Models (HMM). Probabilistic Suffix Trees (PST) were first introduced in [2] as a universal model for data compr ...
... protein using the information contained in its amino acid sequence [1]. Nowadays, the most popular methods to generate a hypothesis about the function of a protein are BLAST and Hidden Markov Models (HMM). Probabilistic Suffix Trees (PST) were first introduced in [2] as a universal model for data compr ...
Teacher Instructions Lesson 4
... Teacher Preparation Tip: The tRNA/Amino Acid stamping tool used in this portion of the lesson needs to be created prior to this segment of instruction. The teacher should make these tools as part of the Teacher Preparation instructions in Lesson 1. Another option would be to have more advanced stude ...
... Teacher Preparation Tip: The tRNA/Amino Acid stamping tool used in this portion of the lesson needs to be created prior to this segment of instruction. The teacher should make these tools as part of the Teacher Preparation instructions in Lesson 1. Another option would be to have more advanced stude ...
Review Problems for amino acids, carbohydrates, glycolysis and the
... is? (there are actually two major compounds formed, one of which you should know). 2- Is this compound an acid, or a base? 3- The normal end point of glycolysis is the formation of pyruvate. This is not the case under conditions of anaerobic exercise- why not? (Consider the various fates of pyruvate ...
... is? (there are actually two major compounds formed, one of which you should know). 2- Is this compound an acid, or a base? 3- The normal end point of glycolysis is the formation of pyruvate. This is not the case under conditions of anaerobic exercise- why not? (Consider the various fates of pyruvate ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.