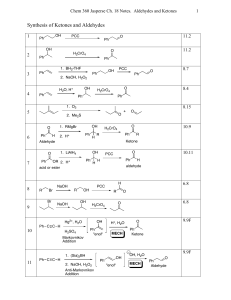
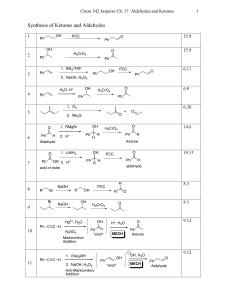
CrO3/Al2O3: Rapid oxidation of alcohols to carbonyl compounds in
... and efficient method the alcohols were oxidized to the corresponding carbonyl compounds when the former was ground in a mortar with a pestle in the presence of supported CrO3 on Al2O3 and a few drops of tBuOH (Scheme I). The feasibility of the oxidation was first examined using benzyl alcohol as a m ...
... and efficient method the alcohols were oxidized to the corresponding carbonyl compounds when the former was ground in a mortar with a pestle in the presence of supported CrO3 on Al2O3 and a few drops of tBuOH (Scheme I). The feasibility of the oxidation was first examined using benzyl alcohol as a m ...
Document
... a yellow or orange precipitate will be formed. ● If the cpd. is insoluble in water, dissolve it in 1 mL of methanol & then add the reagent. 2- Differentiation between aldehydes and ketones: Differentiation between aldehydes and ketones is achieved by taking the advantage of the fact that aldehydes c ...
... a yellow or orange precipitate will be formed. ● If the cpd. is insoluble in water, dissolve it in 1 mL of methanol & then add the reagent. 2- Differentiation between aldehydes and ketones: Differentiation between aldehydes and ketones is achieved by taking the advantage of the fact that aldehydes c ...
O R` R
... • The phosphorus ylides are easily prepared from triphenylphoshine (an excellent nucleophile and weak base) with 1º and 2 º alkyl halides via an SN2 mechanism. • A strong base (usually an alkyllithium or phenyllithium) is required to remove a proton from the intermediate ...
... • The phosphorus ylides are easily prepared from triphenylphoshine (an excellent nucleophile and weak base) with 1º and 2 º alkyl halides via an SN2 mechanism. • A strong base (usually an alkyllithium or phenyllithium) is required to remove a proton from the intermediate ...
Organic Chemistry Lecture Outline Carbonyl
... substitution reactions. Carbonyl groups bonded to large, bulky substituents (eg., tertiary carbons) react slower with nucleophiles than carbonyl groups with smaller, less bulky substituents. Electronic Effects Electronic effects influence the reactivity of the electrophilic carbonyl carbon in nucleo ...
... substitution reactions. Carbonyl groups bonded to large, bulky substituents (eg., tertiary carbons) react slower with nucleophiles than carbonyl groups with smaller, less bulky substituents. Electronic Effects Electronic effects influence the reactivity of the electrophilic carbonyl carbon in nucleo ...
EXPERIMENT 6 (Organic Chemistry II) Pahlavan/Cherif
... Aldehydes and ketones share the carbonyl functional group which features carbon doubly bonded to oxygen. In the case of ketones there are two carbon atoms bonded to the carbonyl carbon and no hydrogens. In the case of aldehydes there is at least one hydrogen bonded to the carbonyl carbon, the other ...
... Aldehydes and ketones share the carbonyl functional group which features carbon doubly bonded to oxygen. In the case of ketones there are two carbon atoms bonded to the carbonyl carbon and no hydrogens. In the case of aldehydes there is at least one hydrogen bonded to the carbonyl carbon, the other ...
Carbonyl Compounds Prior Knowledge
... be able to apply IUPAC rules for nomenclature to alcohols, aldehydes, ketones and carboxylic acids limited to chains with up to 6 carbon atoms understand that alcohols can be classified as primary, secondary or tertiary understand that tertiary alcohols are not easily oxidised understand that primar ...
... be able to apply IUPAC rules for nomenclature to alcohols, aldehydes, ketones and carboxylic acids limited to chains with up to 6 carbon atoms understand that alcohols can be classified as primary, secondary or tertiary understand that tertiary alcohols are not easily oxidised understand that primar ...
Novel amine-catalysed hydroalkoxylation reactions of
... DMAP12 gave smooth conversion to acetal 24, which was isolated in high yield after chromatography (Scheme 3). To our knowledge this is the first example of a nucleophile-promoted dihydroalkoxylation reaction to be reported.13 In summary, it has been found that tertiary nucleophilic amines such as DB ...
... DMAP12 gave smooth conversion to acetal 24, which was isolated in high yield after chromatography (Scheme 3). To our knowledge this is the first example of a nucleophile-promoted dihydroalkoxylation reaction to be reported.13 In summary, it has been found that tertiary nucleophilic amines such as DB ...
Mechanism
... There are two different transformations referred as the Ullmann Reaction. The "classic" Ullmann Reaction is the synthesis of symmetric biaryls via copper-catalyzed coupling. The "Ullmanntype" Reactions include copper-catalyzed Nucleophilic Aromatic Substitution between various nucleophiles (e.g. sub ...
... There are two different transformations referred as the Ullmann Reaction. The "classic" Ullmann Reaction is the synthesis of symmetric biaryls via copper-catalyzed coupling. The "Ullmanntype" Reactions include copper-catalyzed Nucleophilic Aromatic Substitution between various nucleophiles (e.g. sub ...
Chapter 18 – Carbonyl Compounds II (Last Chapter we mostly talk
... to protect a functional group from reacting is quite common in organic chemistry.) (Because they protect functional groups from reacting the groups that are added are called protecting groups. So in the case above, the ketal would be ...
... to protect a functional group from reacting is quite common in organic chemistry.) (Because they protect functional groups from reacting the groups that are added are called protecting groups. So in the case above, the ketal would be ...
Aldol reaction
The aldol reaction is a means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Borodin in 1872, the reaction combines two carbonyl compounds (the original experiments used aldehydes) to form a new β-hydroxy carbonyl compound. These products are known as aldols, from the aldehyde + alcohol, a structural motif seen in many of the products. Aldol structural units are found in many important molecules, whether naturally occurring or synthetic.For example, the aldol reaction has been used in the large-scale production of the commodity chemical pentaerythritoland the synthesis of the heart disease drug Lipitor (atorvastatin, calcium salt).The aldol reaction unites two relatively simple molecules into a more complex one. Increased complexity arises because up to two new stereogenic centers (on the α- and β-carbon of the aldol adduct, marked with asterisks in the scheme below) are formed. Modern methodology is capable of not only allowing aldol reactions to proceed in high yield but also controlling both the relative and absolute stereochemical configuration of these stereocenters. This ability to selectively synthesize a particular stereoisomer is significant because different stereoisomers can have very different chemical and biological properties.For example, stereogenic aldol units are especially common in polyketides, a class of molecules found in biological organisms. In nature, polyketides are synthesized by enzymes that effect iterative Claisen condensations. The 1,3-dicarbonyl products of these reactions can then be variously derivatized to produce a wide variety of interesting structures. Often, such derivitization involves the reduction of one of the carbonyl groups, producing the aldol subunit. Some of these structures have potent biological properties: the immunosuppressant FK506, the anti-tumor agent discodermolide, or the antifungal agent amphotericin B, for example. Although the synthesis of many such compounds was once considered nearly impossible, aldol methodology has allowed their efficient synthesis in many cases.A typical modern aldol addition reaction, shown above, might involve the nucleophilic addition of a ketone enolate to an aldehyde. Once formed, the aldol product can sometimes lose a molecule of water to form an α,β-unsaturated carbonyl compound. This is called aldol condensation. A variety of nucleophiles may be employed in the aldol reaction, including the enols, enolates, and enol ethers of ketones, aldehydes, and many other carbonyl compounds. The electrophilic partner is usually an aldehyde or ketone (many variations, such as the Mannich reaction, exist). When the nucleophile and electrophile are different, the reaction is called a crossed aldol reaction; on the converse, when the nucleophile and electrophile are the same, the reaction is called an aldol dimerization.