
Text - Reading`s CentAUR
... equimolar amounts of Ln(NO3)3 and CyMe4-BTPhen. The only exception was found during the synthesis of the Pr3+ complex of CyMe4-BTPhen, where the major product consisted of a 1:2 Pr/CyMe4-BTPhen complex cation but with a [Pr(NO3)5]2− counterion present per cationic unit. The initial crystallization o ...
... equimolar amounts of Ln(NO3)3 and CyMe4-BTPhen. The only exception was found during the synthesis of the Pr3+ complex of CyMe4-BTPhen, where the major product consisted of a 1:2 Pr/CyMe4-BTPhen complex cation but with a [Pr(NO3)5]2− counterion present per cationic unit. The initial crystallization o ...
Bonding Capabilities of Transition Metal
... the b2 level. The direction of the splitting of the octahedral away from the remaining apical ligand. t2g set, namely b2 below e, is understandable when one Having the energy and shape of the significant molecular considers that the e set has lost some x-bonding interaction orbitals of a flat square ...
... the b2 level. The direction of the splitting of the octahedral away from the remaining apical ligand. t2g set, namely b2 below e, is understandable when one Having the energy and shape of the significant molecular considers that the e set has lost some x-bonding interaction orbitals of a flat square ...
Homogeneous Chromium Catalysts for Olefin Polymerization
... have resulted from many spectroscopic investigations of heterogeneous catalysts combined with extrapolations from the now extensive body of known organometallic structures. However, the relevance of this “deluge of conjecture”[8] is highly questionable. Most of the analytical work utilized IR spectr ...
... have resulted from many spectroscopic investigations of heterogeneous catalysts combined with extrapolations from the now extensive body of known organometallic structures. However, the relevance of this “deluge of conjecture”[8] is highly questionable. Most of the analytical work utilized IR spectr ...
Excited States of Pt(PF3)4 and Their Role in Focused Electron Beam
... factor of 2. The addition of zero point energy slightly lowers the BDE; it is a correction that favors dissociation. Relevance of the included dispersion for the calculation of BDE with the B3LYP functional is important for the analogue complex of Ni(0). BDE of Ni(PF3)4, calculated at the SR-B3LYP-D ...
... factor of 2. The addition of zero point energy slightly lowers the BDE; it is a correction that favors dissociation. Relevance of the included dispersion for the calculation of BDE with the B3LYP functional is important for the analogue complex of Ni(0). BDE of Ni(PF3)4, calculated at the SR-B3LYP-D ...
5.5 Reactions of Inorganic Compounds in - A
... With Co(H2O)4(OH)2 the substitution is complete: [Co(H2O)6]2+(aq) + 2NH3(aq) == [Co(OH)2(H2O)4](s) + 2NH4+(aq) Pink solution blue precipitate [Co(H2O)4(OH)2](s) + 6NH3(aq) == [Co(NH3)6]2+(aq) + 4H2O(l) + 2OH-(aq) blue precipate straw-coloured solution The complex [Co(NH3)6]2+ is oxidised to [Co( ...
... With Co(H2O)4(OH)2 the substitution is complete: [Co(H2O)6]2+(aq) + 2NH3(aq) == [Co(OH)2(H2O)4](s) + 2NH4+(aq) Pink solution blue precipitate [Co(H2O)4(OH)2](s) + 6NH3(aq) == [Co(NH3)6]2+(aq) + 4H2O(l) + 2OH-(aq) blue precipate straw-coloured solution The complex [Co(NH3)6]2+ is oxidised to [Co( ...
Electron transport via metalloporphyrins C.
... cobalt(IV) or a n-dication of cobalt(Il). One might be inclined to conclude that" each of these descriptions are extreme resonance forms of the same molecule and that the molecule should be described as some contribution of all of them. This is not, however, th::: case since the epr spectrum of [Co( ...
... cobalt(IV) or a n-dication of cobalt(Il). One might be inclined to conclude that" each of these descriptions are extreme resonance forms of the same molecule and that the molecule should be described as some contribution of all of them. This is not, however, th::: case since the epr spectrum of [Co( ...
one pot template synthesis and characterization of trivalent transition
... have been confirmed with the help of X-ray measurements32. Thus keeping in view, the analytical data and electrolytic nature of these complexes, a five coordinated square pyramidal geometry may be assigned for these complexes. Thus, assuming the symmetry C4V for these complexes 33, the various spect ...
... have been confirmed with the help of X-ray measurements32. Thus keeping in view, the analytical data and electrolytic nature of these complexes, a five coordinated square pyramidal geometry may be assigned for these complexes. Thus, assuming the symmetry C4V for these complexes 33, the various spect ...
Why does nature use zinc–a personal view†
... A typical textbook entry for each metal is the table of coordination numbers and geometries. This is where for zinc we find a classical textbook error: the Zn(NH3 )6 2+ complex. It has to exist because every decent metal forms a hexammine complex, and as such it appears in about a dozen older publica ...
... A typical textbook entry for each metal is the table of coordination numbers and geometries. This is where for zinc we find a classical textbook error: the Zn(NH3 )6 2+ complex. It has to exist because every decent metal forms a hexammine complex, and as such it appears in about a dozen older publica ...
C-H and H-H Activation in Transition Metal Complexes and on
... source of barriers to C-H activation. In addition to these three interactions, which operate for an ML, complex as well as for a metal surface, there is another interaction, @, which is generally important only for metal surfaces, where there are many closely spaced levels. What happens on the surfa ...
... source of barriers to C-H activation. In addition to these three interactions, which operate for an ML, complex as well as for a metal surface, there is another interaction, @, which is generally important only for metal surfaces, where there are many closely spaced levels. What happens on the surfa ...
Dihalogeno(diphosphane)metal(II) complexes (metal Co, Ni, Pd) as
... to be correctly formulated as [Pd(dppp)(CB11H12)][CB11H11.78(OH)0.22]. The carbon atom in CB11H11Cl in 15 and in the bound and free cage of CB11H12 [or CB11H12(O0.22)] in 16 were located as their B–C bond lengths are slightly shorter than those for B–B. For 15 B–C = 1.701(6)–1.728(8) Å, B–B = 1.733( ...
... to be correctly formulated as [Pd(dppp)(CB11H12)][CB11H11.78(OH)0.22]. The carbon atom in CB11H11Cl in 15 and in the bound and free cage of CB11H12 [or CB11H12(O0.22)] in 16 were located as their B–C bond lengths are slightly shorter than those for B–B. For 15 B–C = 1.701(6)–1.728(8) Å, B–B = 1.733( ...
this PDF file - Journal of the Indian Institute of Science
... alkynes and strained cyclic ole fin^“^. AS vinylidene complexes are also structurally, in part, metallacarbenes, they should be able to initiate such polymerisation reactions. It should also be noted that stable vinylidene complexes may not be good catalysts. For instance, the cationic complex conta ...
... alkynes and strained cyclic ole fin^“^. AS vinylidene complexes are also structurally, in part, metallacarbenes, they should be able to initiate such polymerisation reactions. It should also be noted that stable vinylidene complexes may not be good catalysts. For instance, the cationic complex conta ...
Near-Infrared Luminescent Lanthanide Complexes
... resulting in long and expensive amplifiers. A promising strategy to overcome these drawbacks is to encapsulate the lanthanide ion by a “light harvesting” organic ligand which allows optical pumping and provides good solubility in the host material. Moreover, the ligand can shield the ion from additi ...
... resulting in long and expensive amplifiers. A promising strategy to overcome these drawbacks is to encapsulate the lanthanide ion by a “light harvesting” organic ligand which allows optical pumping and provides good solubility in the host material. Moreover, the ligand can shield the ion from additi ...
Chapter 12 EDTA Titrations Coordination Number Geometries Ligands
... There are two common geometries for metals with a coordination number of four: Tetrahedral & Square planar ...
... There are two common geometries for metals with a coordination number of four: Tetrahedral & Square planar ...
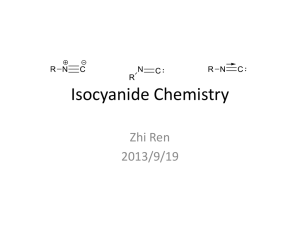
Isocyanide Chemistry
... • The first isocyanide complex Ag(CNR)(CN) was obtained by Gautierin 1869. • But until 1977, the first zero-valent isocyanide complexes Co2(CNR)8 were obtained. • Now, a large number of isocyanide complexes involving metals from Group 5 to Group 11 and Ti. ...
... • The first isocyanide complex Ag(CNR)(CN) was obtained by Gautierin 1869. • But until 1977, the first zero-valent isocyanide complexes Co2(CNR)8 were obtained. • Now, a large number of isocyanide complexes involving metals from Group 5 to Group 11 and Ti. ...
CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS
... B01J 29/86 - B01J 29/89 and B01J 29/046 - B01J 29/048 take precedence. The use of catalysts must also be classified in the appropriate groups, such as in C01B, C01C, C10G, C07B, C07C, C07D ...
... B01J 29/86 - B01J 29/89 and B01J 29/046 - B01J 29/048 take precedence. The use of catalysts must also be classified in the appropriate groups, such as in C01B, C01C, C10G, C07B, C07C, C07D ...
A Metal-Based Inhibitor of Tumor Necrosis Factor-α
... has not yet been explored. As demonstrated recently by the group of Meggers, kinetically inert octahedral metal complexes can be utilized as structurally diverse molecular scaffolds for the design of potent and selective enzyme inhibitors.[12] Herein, we have pursued the investigation of the cyclome ...
... has not yet been explored. As demonstrated recently by the group of Meggers, kinetically inert octahedral metal complexes can be utilized as structurally diverse molecular scaffolds for the design of potent and selective enzyme inhibitors.[12] Herein, we have pursued the investigation of the cyclome ...
mod-5-revision-guide-4-transition-metals
... Cr3+ (green) and then Cr2+ (blue) are formed by reduction of Cr2O72- (orange) by the strong reducing agent zinc in (HCl) acid solution. Fe2+ is a less strong reducing agent and will only reduce the dichromate to Cr3+ . The Fe2+ and Cr2O7 2- in acid solution reaction can be used as a quantitative red ...
... Cr3+ (green) and then Cr2+ (blue) are formed by reduction of Cr2O72- (orange) by the strong reducing agent zinc in (HCl) acid solution. Fe2+ is a less strong reducing agent and will only reduce the dichromate to Cr3+ . The Fe2+ and Cr2O7 2- in acid solution reaction can be used as a quantitative red ...
pdf - 283KB
... (a) To enclose sets of identical groups of atoms (the entity may be an ion, substituent group, or molecule). Usually a multiplicative subscript follows the closing parenthesis. In the case of common ions such as nitrate and sulfate parentheses are recommended but not mandatory. ...
... (a) To enclose sets of identical groups of atoms (the entity may be an ion, substituent group, or molecule). Usually a multiplicative subscript follows the closing parenthesis. In the case of common ions such as nitrate and sulfate parentheses are recommended but not mandatory. ...
Synthesis and Characterization of Bis-Phosphine
... In addition to classifying ligands according to the number of donor atoms and/or configuration, they may also be classified according to the polarizability of the donor atom(s), again utilizing Pearson’s classification scheme.9 According to Pearson, species which are more easily polarizable such as, ...
... In addition to classifying ligands according to the number of donor atoms and/or configuration, they may also be classified according to the polarizability of the donor atom(s), again utilizing Pearson’s classification scheme.9 According to Pearson, species which are more easily polarizable such as, ...
Evolution of strategies to prepare synthetic mimics of carboxylate
... after ~10 min. The UV-visible (UV-vis), Mössbauer, resonance Raman, and EXAFS spectra of the transient intermediate closely match those of oxyHr (Scheme 1, top), strongly suggesting that 11 contains a (hydroperoxo)(µ-oxo)diiron(III) unit. Unlike Hr, however, oxygenation of 10 is irreversible and le ...
... after ~10 min. The UV-visible (UV-vis), Mössbauer, resonance Raman, and EXAFS spectra of the transient intermediate closely match those of oxyHr (Scheme 1, top), strongly suggesting that 11 contains a (hydroperoxo)(µ-oxo)diiron(III) unit. Unlike Hr, however, oxygenation of 10 is irreversible and le ...
couverture these PRES Toulouse M ESCARCEGA 2011
... several times through this cycle; in this sense, the catalyst remains unaltered but dynamic. The number of moles of substrate that a mole of catalyst can convert into product molecules through this cycle before becoming inactivated is called the turnover number (TON) and is a measure of the catalys ...
... several times through this cycle; in this sense, the catalyst remains unaltered but dynamic. The number of moles of substrate that a mole of catalyst can convert into product molecules through this cycle before becoming inactivated is called the turnover number (TON) and is a measure of the catalys ...
On the Electronic Structure of [1Fe] Fe−S Complexes from Anionic
... analogues were all ferrous complexes in the solution. When the samples were sprayed in ambient atmosphere, however, the ferrous complexes could be oxidized in the ESI source. Thus both ferrous and ferric species were observed in the mass spectra. Photodetachment Photoelectron Spectroscopy. Details o ...
... analogues were all ferrous complexes in the solution. When the samples were sprayed in ambient atmosphere, however, the ferrous complexes could be oxidized in the ESI source. Thus both ferrous and ferric species were observed in the mass spectra. Photodetachment Photoelectron Spectroscopy. Details o ...
Inorganic Chemistry - Name
... "Beyond this I have not the time or the inclination to go?" The, answer to this question must of course vary with the special field of experimental work and with the individual. In some areas fairly advanced theory is indispensable. In others relatively little is useful. For the most part, however, ...
... "Beyond this I have not the time or the inclination to go?" The, answer to this question must of course vary with the special field of experimental work and with the individual. In some areas fairly advanced theory is indispensable. In others relatively little is useful. For the most part, however, ...
Ligand
4-3D-balls.png?width=300)
In coordination chemistry, a ligand (/lɪɡənd/) is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from covalent to ionic. Furthermore, the metal-ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic ""ligand.""Metals and metalloids are bound to ligands in virtually all circumstances, although gaseous ""naked"" metal ions can be generated in high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection is a critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chemistry.Ligands are classified in many ways like : their charge, their size (bulk), the identity of the coordinating atom(s), and the number of electrons donated to the metal (denticity or hapticity). The size of a ligand is indicated by its cone angle.





















![On the Electronic Structure of [1Fe] Fe−S Complexes from Anionic](http://s1.studyres.com/store/data/016662851_1-146ca92fb5881f9927dee1ea56bc2f4e-300x300.png)
