CROSS-COUPLING REACTIONS OF ORGANOBORANES: AN EASY WAY FOR C-C BONDING
... Mechanism of the Vinylic-Vinylic Cross-Coupling The principal features of the cross-coupling reaction are as follows: (a) Small catalytic amounts of palladium complexes (1–3 mol %) are required to obtain the coupled products. (b) The coupling reactions are highly regioand stereoselective and take pl ...
... Mechanism of the Vinylic-Vinylic Cross-Coupling The principal features of the cross-coupling reaction are as follows: (a) Small catalytic amounts of palladium complexes (1–3 mol %) are required to obtain the coupled products. (b) The coupling reactions are highly regioand stereoselective and take pl ...
Document
... Diazomethane forms methylene, which converts alkenes into cyclopropanes. The highly reactive species methylene, H2C: (the simplest carbene) can be produced from the decomposition of diazomethane: ...
... Diazomethane forms methylene, which converts alkenes into cyclopropanes. The highly reactive species methylene, H2C: (the simplest carbene) can be produced from the decomposition of diazomethane: ...
Chapter One: Molecular Structure
... reaction between ethers and epoxides with nucleophiles under acidic and basic conditions. Predict the stereochemistry and optical activity of a product from an understanding of its mechanism of formation. Propose a reaction or sequence of reactions to produce a target ether or epoxide in high yi ...
... reaction between ethers and epoxides with nucleophiles under acidic and basic conditions. Predict the stereochemistry and optical activity of a product from an understanding of its mechanism of formation. Propose a reaction or sequence of reactions to produce a target ether or epoxide in high yi ...
Chapter 7
... atoms in the T.S. of an E2 reaction must lie in the same plane. • There are two ways this can happen: ...
... atoms in the T.S. of an E2 reaction must lie in the same plane. • There are two ways this can happen: ...
Document
... In acidic or basic solution, aldehydes can undergo condensations, reactions in which molecules combine to yield larger molecules. With aldehydes, this particular type of condensation reaction is called an aldol condensation, or an aldol addition, because the product is both an aldehyde and an alcoho ...
... In acidic or basic solution, aldehydes can undergo condensations, reactions in which molecules combine to yield larger molecules. With aldehydes, this particular type of condensation reaction is called an aldol condensation, or an aldol addition, because the product is both an aldehyde and an alcoho ...
Table
... With Nitric Acid Complete combustion reactions Addition Reactions With H2: Hydrogenation With halogens or hydrogen halides: Halogenation With water: Hydration Substitution Reactions With X2: Halogenation Ø-X With HNO3: Nitration Ø-NO2 With RX: Alkylation Ø-R Preparation Alkenes and alkynes Organi ...
... With Nitric Acid Complete combustion reactions Addition Reactions With H2: Hydrogenation With halogens or hydrogen halides: Halogenation With water: Hydration Substitution Reactions With X2: Halogenation Ø-X With HNO3: Nitration Ø-NO2 With RX: Alkylation Ø-R Preparation Alkenes and alkynes Organi ...
Organic Chemistry
... KMnO4, in one case only, the purple colour disappears and brown ppt. is formed. Ans1. Only the n-butanol would be oxidised by dil. KMnO4 solution and the brown ppt. formed is of MnO2, manganese oxide. KMnO4 + C4H9OH 2KOH + 2MnO2 + C3H7COOH Q2. Explain why Dry gaseous hydrohalic acids and not their ...
... KMnO4, in one case only, the purple colour disappears and brown ppt. is formed. Ans1. Only the n-butanol would be oxidised by dil. KMnO4 solution and the brown ppt. formed is of MnO2, manganese oxide. KMnO4 + C4H9OH 2KOH + 2MnO2 + C3H7COOH Q2. Explain why Dry gaseous hydrohalic acids and not their ...
Chapter Nine: Alcohols, Ethers and Epoxides
... reaction with ethers and their mechanism of formation. Predict the stereochemistry and optical activity of a product from an understanding of its mechanism of formation. Propose a reaction or sequence of reactions to produce a target ether, alcohol or epoxide in high yield. Predict the product ...
... reaction with ethers and their mechanism of formation. Predict the stereochemistry and optical activity of a product from an understanding of its mechanism of formation. Propose a reaction or sequence of reactions to produce a target ether, alcohol or epoxide in high yield. Predict the product ...
Redox Reactions
... Alkali metal reductions are used for the stereochemistry of the reduction: the addition of an electron to each side of the double bond forces a “trans” configuraton of the addition of H2. Na0 NH3, -78°C ...
... Alkali metal reductions are used for the stereochemistry of the reduction: the addition of an electron to each side of the double bond forces a “trans” configuraton of the addition of H2. Na0 NH3, -78°C ...
Lecture Notes 12 - La Salle University
... In the trigonal plane of the 5-coordinate transition state or intermediate, a πbonding interaction can occur between a metal d-orbital (e.g. dxy) and suitable orbitals (p atomic orbitals, or molecular orbitals of p-symmetry) of ligand L2 (the ligand trans to the leaving group) and Y (the entering gr ...
... In the trigonal plane of the 5-coordinate transition state or intermediate, a πbonding interaction can occur between a metal d-orbital (e.g. dxy) and suitable orbitals (p atomic orbitals, or molecular orbitals of p-symmetry) of ligand L2 (the ligand trans to the leaving group) and Y (the entering gr ...
Chapter 7 Alkenes and Alkynes I
... Syn Addition of Hydrogen: Synthesis of cis-Alkenes The P-2 catalyst nickel boride results in syn addition of one equivalent of hydrogen to a triple bond An internal alkyne will yield a cis double bond ...
... Syn Addition of Hydrogen: Synthesis of cis-Alkenes The P-2 catalyst nickel boride results in syn addition of one equivalent of hydrogen to a triple bond An internal alkyne will yield a cis double bond ...
Activity 5 – Catalytic Cycles
... We will look at a number of catalytic cycles below, but first it is useful to think about the key steps which make up all the catalytic cycles. 1) Oxidative Addition (Figure 7). In this step the metal in the centre loses electrons to the ligands as the ligand are added to the metal centre, hence the ...
... We will look at a number of catalytic cycles below, but first it is useful to think about the key steps which make up all the catalytic cycles. 1) Oxidative Addition (Figure 7). In this step the metal in the centre loses electrons to the ligands as the ligand are added to the metal centre, hence the ...
Organometallic Compounds - Reagents
... Alcoholic solvents and water are incompatible with Grignard reagents and organolithium reagents. Reactivity of the alkyl halide: -I > -Br > -Cl >> -F alkyl halides > vinyl or aryl halides ...
... Alcoholic solvents and water are incompatible with Grignard reagents and organolithium reagents. Reactivity of the alkyl halide: -I > -Br > -Cl >> -F alkyl halides > vinyl or aryl halides ...
Study_guide_2010-01
... listed in italics. PLTL topics are in CAPS. This document will be updated throughout the term. The goals of this course are: - to achieve an advanced understanding of the reactivity of organic molecules - to understand the sources of selective reactions on complex organic molecules - to learn the ba ...
... listed in italics. PLTL topics are in CAPS. This document will be updated throughout the term. The goals of this course are: - to achieve an advanced understanding of the reactivity of organic molecules - to understand the sources of selective reactions on complex organic molecules - to learn the ba ...
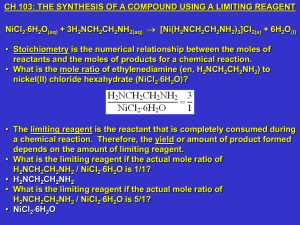
Week #10: The Synthesis of a Compound using a Limiting Reagent
... cation bonded (or coordinated) to 1 or more groups of atoms. These groups of atoms are called ligands. • Coordination number is the number of ligand atoms that bond to the central metal of a complex ion. • What is the metal atom in this complex ion? • Ni2+ • What is the ligand? ...
... cation bonded (or coordinated) to 1 or more groups of atoms. These groups of atoms are called ligands. • Coordination number is the number of ligand atoms that bond to the central metal of a complex ion. • What is the metal atom in this complex ion? • Ni2+ • What is the ligand? ...
Document
... cation bonded (or coordinated) to 1 or more groups of atoms. These groups of atoms are called ligands. • Coordination number is the number of ligand atoms that bond to the central metal of a complex ion. • What is the metal atom in this complex ion? • Ni2+ • What is the ligand? ...
... cation bonded (or coordinated) to 1 or more groups of atoms. These groups of atoms are called ligands. • Coordination number is the number of ligand atoms that bond to the central metal of a complex ion. • What is the metal atom in this complex ion? • Ni2+ • What is the ligand? ...
• Pergamon
... 2-methyl group occurred within 5 min. Then, addition of the a-free pyrrole, 2-benzyloxycarbonyl-3,4-dimethylpyrrole 9, into the same reaction mixture resulted in in situ coupling to provide (after ...
... 2-methyl group occurred within 5 min. Then, addition of the a-free pyrrole, 2-benzyloxycarbonyl-3,4-dimethylpyrrole 9, into the same reaction mixture resulted in in situ coupling to provide (after ...
Lecture 8a - UCLA Chemistry and Biochemistry
... electrophilic atoms (i.e., ester, ketone, nitro compounds, sulfoxide) are not suitable • Hydrocarbons are non-polar (or weakly polar) and do not dissolve the moderately polar Grignard reagent well enough • Ethers are most commonly used as single solvent because they are stable and polar enough to di ...
... electrophilic atoms (i.e., ester, ketone, nitro compounds, sulfoxide) are not suitable • Hydrocarbons are non-polar (or weakly polar) and do not dissolve the moderately polar Grignard reagent well enough • Ethers are most commonly used as single solvent because they are stable and polar enough to di ...
... industrial scale, their use is rare. In this respect, the few successful catalysts developed for the enantioselective addition of trialkylaluminium to aldehydes can be grouped in two types. The first group are the titanium complexes that usually afford high enantioselectivities, but the high catalys ...
Calculating Percent Yield
... The electrons from the C-H bond then move down to reform the pi bond and the very stable aromatic ring. ...
... The electrons from the C-H bond then move down to reform the pi bond and the very stable aromatic ring. ...
Grignard Reagents
... 14.9: Retrosynthetic Analysis - the process of planning a synthesis by reasoning backward from the the target molecule to a starting compound using known and reliable reactions. “it is a problem solving technique for transforming the structure of a synthetic target molecule (TM) to a sequence of pr ...
... 14.9: Retrosynthetic Analysis - the process of planning a synthesis by reasoning backward from the the target molecule to a starting compound using known and reliable reactions. “it is a problem solving technique for transforming the structure of a synthetic target molecule (TM) to a sequence of pr ...
Study guide/lecture topics
... listed in italics. PLTL topics are in CAPS. This document will be updated throughout the term. The goals of this course are: - to achieve an advanced understanding of the reactivity of organic molecules - to understand the sources of selective reactions on complex organic molecules - to learn the ba ...
... listed in italics. PLTL topics are in CAPS. This document will be updated throughout the term. The goals of this course are: - to achieve an advanced understanding of the reactivity of organic molecules - to understand the sources of selective reactions on complex organic molecules - to learn the ba ...
Organic Synthesis
... but at least one is required. So, of all the reactions which are available to the organic chemist, we can be sure that we need one of the relatively small group of C-C bond forming reactions. Second, the product must contain an alcohol group. That may have survived from the starting material, or bee ...
... but at least one is required. So, of all the reactions which are available to the organic chemist, we can be sure that we need one of the relatively small group of C-C bond forming reactions. Second, the product must contain an alcohol group. That may have survived from the starting material, or bee ...
Chem+174–Lecture12a
... to ligands like CO, CN, etc. Tolman observed for Ni(CO)3L that the carbonyl stretching frequency decreases as the donor ability of the R-group increases (i.e., PCy3 (2056 cm-1) vs. P(OMe)3 (2070 cm-1) vs. ...
... to ligands like CO, CN, etc. Tolman observed for Ni(CO)3L that the carbonyl stretching frequency decreases as the donor ability of the R-group increases (i.e., PCy3 (2056 cm-1) vs. P(OMe)3 (2070 cm-1) vs. ...
Practice Problem
... dialkylcopper (Gilman reagents) 3. Lithium dialkylcopper reagents react with alkyl halides to give alkanes ...
... dialkylcopper (Gilman reagents) 3. Lithium dialkylcopper reagents react with alkyl halides to give alkanes ...
Stille reaction

The Stille reaction, or the Migita-Kosugi-Stille coupling, is a chemical reaction widely used in organic synthesis which involves the coupling of an organotin compound (also known as organostannanes) with a variety of organic electrophiles via palladium-catalyzed coupling reaction.The R1 group attached to the trialkyltin is normally sp2-hybridized, including alkenes, and aryl groups; however, conditions have been devised to incorporate both sp3-hybridized groups, such as allylic and benzylic substituents, and sp-hybridized alkynes. These organostannanes are also stable to both air and moisture, and many of these reagents are either commercially available or can be synthesized from literature precedent. However, these tin reagents tend to be highly toxic. X is typically a halide, such as Cl, Br, I, yet pseudohalides such as triflates and sulfonates and phosphates can also be used.The groundwork for the Stille reaction was laid by Colin Eaborn, Toshihiko Migita, and Masanori Kosugi in 1976 and 1977, who explored numerous palladium catalyzed couplings involving organotin reagents. John Stille and David Milstein developed a much milder and more broadly applicable procedure in 1978. Stille’s work on this area might have earned him a share of the 2010 Nobel Prize, which was awarded to Richard Heck, Ei-ichi Negishi, and Akira Suzuki for their work on the Heck, Negishi, and Suzuki coupling reactions. However, Stille died in the plane crash of United Airlines Flight 232 in 1989.Several reviews have been published on the Stille reaction.