oxidation and reduction
... Axial alcohols are oxidised faster than equatorial alcohols since the rate determining breakdown of the axial intermediate A is accompanied by relief of 1,3-diaxial interactions not present in the breakdown of E. ...
... Axial alcohols are oxidised faster than equatorial alcohols since the rate determining breakdown of the axial intermediate A is accompanied by relief of 1,3-diaxial interactions not present in the breakdown of E. ...
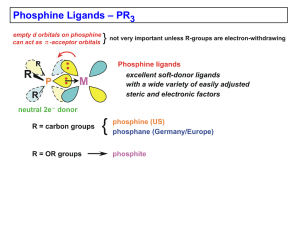
Chapter 4 (Phosphines)
... PMePh2 (136°), PMe2Ph (122°), PMe3 (118°), PEt3 (132°) P(Cy)3 (170°) tricyclohexylphosphine, P(t-Bu)3 (182°) • the alkyl phosphines are strong s-donors; low MW ones usually colorless liquids, somewhat to very air-sensitive, horrible smelling (unless very high MW and nonvolatile) Poor s-Donors, Good ...
... PMePh2 (136°), PMe2Ph (122°), PMe3 (118°), PEt3 (132°) P(Cy)3 (170°) tricyclohexylphosphine, P(t-Bu)3 (182°) • the alkyl phosphines are strong s-donors; low MW ones usually colorless liquids, somewhat to very air-sensitive, horrible smelling (unless very high MW and nonvolatile) Poor s-Donors, Good ...
Enantiodivergent conversion of chiral secondary alcohols into
... •Summary (comparison to other methods) ...
... •Summary (comparison to other methods) ...
NMR Spectroscopy
... D771B Ext. 2314; Email: Dibble@uleth.ca Class we b site (for downloading class materials): classes.uleth.ca/200601/chem2600a/ or follow the links at classes.uleth.ca Prerequisite: Chem 2500 or permission of instructor. If yo u do no t have the prerequisite yo u will be deregistered. Text: "Organic C ...
... D771B Ext. 2314; Email: Dibble@uleth.ca Class we b site (for downloading class materials): classes.uleth.ca/200601/chem2600a/ or follow the links at classes.uleth.ca Prerequisite: Chem 2500 or permission of instructor. If yo u do no t have the prerequisite yo u will be deregistered. Text: "Organic C ...
Reductive Deoxygenation of Ketones and Secondary Alcohols by
... The reductive deoxygenation of ketones and secondary alcohols to the corresponding methylene hydrocarbons has been achieved in good to excellent yield by the combined action of an aluminum hydride source and a strongly Lewis-acidic aluminum reagent. Such reductions were successful with diaryl ketone ...
... The reductive deoxygenation of ketones and secondary alcohols to the corresponding methylene hydrocarbons has been achieved in good to excellent yield by the combined action of an aluminum hydride source and a strongly Lewis-acidic aluminum reagent. Such reductions were successful with diaryl ketone ...
Chapter 10 Outline: Alcohols
... Reaction with Thionyl Halide (SOX2, X = Cl, Br) (converting a ROH →RX) ...
... Reaction with Thionyl Halide (SOX2, X = Cl, Br) (converting a ROH →RX) ...
Chapter 8 Alkenes and Alkynes II: Addition Reactions Alkenes are
... Carbenoids: the Simmon-Smith reaction ...
... Carbenoids: the Simmon-Smith reaction ...
asymmetric alkyne addition to aldehydes
... With the emergence of the Ti-BINOL catalyst system, the effect of substituents about the BINOL core was examined. Different 3,3’-substitued BINOL ligands for the asymmetric addition of alkynes to carbonyl compounds were investigated.14 With 3,3’-substituted BINOL 15 (10 mol %), the addition of pheny ...
... With the emergence of the Ti-BINOL catalyst system, the effect of substituents about the BINOL core was examined. Different 3,3’-substitued BINOL ligands for the asymmetric addition of alkynes to carbonyl compounds were investigated.14 With 3,3’-substituted BINOL 15 (10 mol %), the addition of pheny ...
Origin of the Diastereoselection in the Indium
... We tentatively explain the high and opposite stereoselections for anti-3a and for syn-3n according to the R1 substituent of 1 using the model proposed by Hoffmann.10 Hoffmann connected the conformation of the complex between metal enolate and aldehyde to the stereochemistry of the aldol product thro ...
... We tentatively explain the high and opposite stereoselections for anti-3a and for syn-3n according to the R1 substituent of 1 using the model proposed by Hoffmann.10 Hoffmann connected the conformation of the complex between metal enolate and aldehyde to the stereochemistry of the aldol product thro ...
lecture 7 reductive eliminations
... • In the SN2 pathway, adopted for polarized A‐B substrates such as alkyl halides, the metal electron pair of LnM directly attacks the A–B σ* orbital by an in‐line attack at the least electronegative atom (where σ* is largest) formally to give LnM2+ , A−, and B− fragments (ionic model). ...
... • In the SN2 pathway, adopted for polarized A‐B substrates such as alkyl halides, the metal electron pair of LnM directly attacks the A–B σ* orbital by an in‐line attack at the least electronegative atom (where σ* is largest) formally to give LnM2+ , A−, and B− fragments (ionic model). ...
Materials Seminar Professor Carsten Sievers Georgia Institute of Technology
... with reduced reactivity and corrosiveness and increases the energy density of the product. In HDO, oxygen-containing functional groups are replaced by hydrogen, and water is formed as a by-product. This process can be performed over sulfided NiMo or CoMo catalysts. However, co-feeding of toxic H2S i ...
... with reduced reactivity and corrosiveness and increases the energy density of the product. In HDO, oxygen-containing functional groups are replaced by hydrogen, and water is formed as a by-product. This process can be performed over sulfided NiMo or CoMo catalysts. However, co-feeding of toxic H2S i ...
Synopsis
... Toward this end, debromination of 57 was effected by treatment with ntributyltin hydride in refluxing benzene in the presence of cat. amounts of AIBN to furnish acetonide 58. Deprotection of the acetonide using cat. CSA in methanol proceeded cleanly to yield alcohol 59 which was protected as its ace ...
... Toward this end, debromination of 57 was effected by treatment with ntributyltin hydride in refluxing benzene in the presence of cat. amounts of AIBN to furnish acetonide 58. Deprotection of the acetonide using cat. CSA in methanol proceeded cleanly to yield alcohol 59 which was protected as its ace ...
Microsoft Word
... 30. This is generally prepared by O-methylation of β-naphthol 29 which is carried out by using highly toxic methylating agents such as methyl halides or dimethyl sulfate and a base such as sodium hydroxide. In the present work, we have used dimethyl carbonate as the methylating agent. Dimethyl carbo ...
... 30. This is generally prepared by O-methylation of β-naphthol 29 which is carried out by using highly toxic methylating agents such as methyl halides or dimethyl sulfate and a base such as sodium hydroxide. In the present work, we have used dimethyl carbonate as the methylating agent. Dimethyl carbo ...
Ch 16 Electrophilic Aromatic Substitution
... Notice how the Al atom gains e-1’s in order to fulfill its octet, rather than losing e-1’s as it typically does to become a metal cation. There are limitations on the types of alkyl chlorides and aromatic rings that will work. First the chloride must be alkyl (Cl is attached to an sp3 C), and not vi ...
... Notice how the Al atom gains e-1’s in order to fulfill its octet, rather than losing e-1’s as it typically does to become a metal cation. There are limitations on the types of alkyl chlorides and aromatic rings that will work. First the chloride must be alkyl (Cl is attached to an sp3 C), and not vi ...
Durham Research Online
... of various phosphines (PH3, PMe3, PPh3, P{c-hexyl}3) using ONIOM computational methods.12 These studies explored the two most common reductive elimination pathways, one involving tetra-coordinate complexes and one T-shaped tri-coordinate species, the latter formed by ligand pre-dissociation (Scheme ...
... of various phosphines (PH3, PMe3, PPh3, P{c-hexyl}3) using ONIOM computational methods.12 These studies explored the two most common reductive elimination pathways, one involving tetra-coordinate complexes and one T-shaped tri-coordinate species, the latter formed by ligand pre-dissociation (Scheme ...
Chapter 10
... Grignard reagents are strong nucleophiles so they will react with any electrophilic double bond ...
... Grignard reagents are strong nucleophiles so they will react with any electrophilic double bond ...
Slide 1
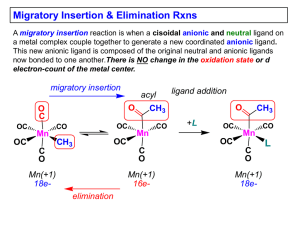
... 1) No change in formal oxidation state (exception: alkylidenes) 2) The two groups that react must be cisoidal to one another 3) A vacant coordination site is generated by the migratory insertion. Therefore, a vacant site is required for the back elimination reaction (e.g., b-hydride elimination). A ...
... 1) No change in formal oxidation state (exception: alkylidenes) 2) The two groups that react must be cisoidal to one another 3) A vacant coordination site is generated by the migratory insertion. Therefore, a vacant site is required for the back elimination reaction (e.g., b-hydride elimination). A ...
(1) and New York University (2)
... We intend to use these ligands to further develop circularly polarized fluorescence excitation (CPE), which is based on fluorescence-detected circular dichroism, which gives better contrast and eliminates many spectral interferences. ...
... We intend to use these ligands to further develop circularly polarized fluorescence excitation (CPE), which is based on fluorescence-detected circular dichroism, which gives better contrast and eliminates many spectral interferences. ...
Chemdraw B&W - Pennsylvania State University
... • Sodium hydride (NaH) or lithium diisopropylamide [LiN(i-C3H7)2] are strong enough to form the enolate ...
... • Sodium hydride (NaH) or lithium diisopropylamide [LiN(i-C3H7)2] are strong enough to form the enolate ...
Poly(ethylene glycol)-supported a,a,a
... effective catalyst in dioxirane mediated alkene epoxidation reactions and that is approximately as efficient as is the analogous small molecule ketone, a,a,a-trifluoroacetophenone.18,33,34 Due to its solubility, 2 functions as a homogeneous catalyst and, therefore, allows for much shorter reaction times ...
... effective catalyst in dioxirane mediated alkene epoxidation reactions and that is approximately as efficient as is the analogous small molecule ketone, a,a,a-trifluoroacetophenone.18,33,34 Due to its solubility, 2 functions as a homogeneous catalyst and, therefore, allows for much shorter reaction times ...
Reaction Rate review questions
... Show that the sum of the two steps in the reaction mechanism is the same as the overall equation for the reaction. What is the rate-determining step? Explain. First step is the slowest so it is the rate determining step. Identify any intermediates or catalysts. F is an intermediate, no catalyst. Pre ...
... Show that the sum of the two steps in the reaction mechanism is the same as the overall equation for the reaction. What is the rate-determining step? Explain. First step is the slowest so it is the rate determining step. Identify any intermediates or catalysts. F is an intermediate, no catalyst. Pre ...
Grant MacEwan College - Faculty Web Pages
... instructor(s) of any special needs that are identified. See Policy E3400 Students with Disabilities (found here). 12. Student Appeals: The University has a policy regarding Student Appeals (E3103, found here). You should access this policy to become aware of the deadlines and guidelines that need to ...
... instructor(s) of any special needs that are identified. See Policy E3400 Students with Disabilities (found here). 12. Student Appeals: The University has a policy regarding Student Appeals (E3103, found here). You should access this policy to become aware of the deadlines and guidelines that need to ...
Asymmetric (stereoselective) synthesis
... • Stereoselective reactions — reactions where one stereoisomer of product is formed predominantly because the reaction has a choice of pathways, and one pathway is more favourable than the other • Selective reduction of 4-tert-butylcyclohexanone (I) to a 10:1 mixture of trans- and cis-4-tert-butylcy ...
... • Stereoselective reactions — reactions where one stereoisomer of product is formed predominantly because the reaction has a choice of pathways, and one pathway is more favourable than the other • Selective reduction of 4-tert-butylcyclohexanone (I) to a 10:1 mixture of trans- and cis-4-tert-butylcy ...
Retrosynthesis - Organic Chemistry
... • Lewis acid base theory is extremely useful in PREDICTING the products of organic reactions • Although it works as a fundamental theory, we will find that occasionally we have to just "know" some reagents, the same will apply for reaction in reverse, next……. ...
... • Lewis acid base theory is extremely useful in PREDICTING the products of organic reactions • Although it works as a fundamental theory, we will find that occasionally we have to just "know" some reagents, the same will apply for reaction in reverse, next……. ...
Stille reaction

The Stille reaction, or the Migita-Kosugi-Stille coupling, is a chemical reaction widely used in organic synthesis which involves the coupling of an organotin compound (also known as organostannanes) with a variety of organic electrophiles via palladium-catalyzed coupling reaction.The R1 group attached to the trialkyltin is normally sp2-hybridized, including alkenes, and aryl groups; however, conditions have been devised to incorporate both sp3-hybridized groups, such as allylic and benzylic substituents, and sp-hybridized alkynes. These organostannanes are also stable to both air and moisture, and many of these reagents are either commercially available or can be synthesized from literature precedent. However, these tin reagents tend to be highly toxic. X is typically a halide, such as Cl, Br, I, yet pseudohalides such as triflates and sulfonates and phosphates can also be used.The groundwork for the Stille reaction was laid by Colin Eaborn, Toshihiko Migita, and Masanori Kosugi in 1976 and 1977, who explored numerous palladium catalyzed couplings involving organotin reagents. John Stille and David Milstein developed a much milder and more broadly applicable procedure in 1978. Stille’s work on this area might have earned him a share of the 2010 Nobel Prize, which was awarded to Richard Heck, Ei-ichi Negishi, and Akira Suzuki for their work on the Heck, Negishi, and Suzuki coupling reactions. However, Stille died in the plane crash of United Airlines Flight 232 in 1989.Several reviews have been published on the Stille reaction.