Organic_chemistry
... Homologous series • The ability of carbon atoms to form chains leads to the existence of a series of compounds that have the • same functional group (and hence similar chemical properties) and • only differ from each other by the presence of an additional carbon atom and its two associated hydroge ...
... Homologous series • The ability of carbon atoms to form chains leads to the existence of a series of compounds that have the • same functional group (and hence similar chemical properties) and • only differ from each other by the presence of an additional carbon atom and its two associated hydroge ...
Nomenclature of Polyfunctional Organic Compounds
... name must be strictly systematic so that it can be assigned and interpreted by computers; common names are not allowed. People,however,have different requirements than computers. For peoplewhich is to say chemists in their spoken and written communications-it's best that a chemical name be pronounce ...
... name must be strictly systematic so that it can be assigned and interpreted by computers; common names are not allowed. People,however,have different requirements than computers. For peoplewhich is to say chemists in their spoken and written communications-it's best that a chemical name be pronounce ...
chapter20(10-27-14)
... • In primary amines, only one of the hydrogen atoms in the ammonia molecule has been replaced. That means that the formula of the primary amine will be R-NH2 where "R" is an alkyl group. • Examples include: ...
... • In primary amines, only one of the hydrogen atoms in the ammonia molecule has been replaced. That means that the formula of the primary amine will be R-NH2 where "R" is an alkyl group. • Examples include: ...
IB2 SL CHEMISTRY Name: …………………………… Topic 10
... Which statement about successive members of all homologous series is correct? ...
... Which statement about successive members of all homologous series is correct? ...
Compounds and molecules: - Wikispaces
... • Some compounds are made of molecules. For example, Sugar-C12H22O11 is molecule. Like, oxygen-O2, Nitrogen-N2 are molecular compounds. • Very weak force of attraction is existing between molecules, they exist as solids, liquids or gases. Melting points and boiling points depend on their physical st ...
... • Some compounds are made of molecules. For example, Sugar-C12H22O11 is molecule. Like, oxygen-O2, Nitrogen-N2 are molecular compounds. • Very weak force of attraction is existing between molecules, they exist as solids, liquids or gases. Melting points and boiling points depend on their physical st ...
Chapter 22 HEIN
... the final –e of the corresponding alkane by –ol. When isomers are possible, locate the position of the –OH group by placing the number (hyphenated) of the carbon atom to which the –OH is bonded immediately before the parent alcohol name. 4. Name each alkyl side chain (or other group), and designate ...
... the final –e of the corresponding alkane by –ol. When isomers are possible, locate the position of the –OH group by placing the number (hyphenated) of the carbon atom to which the –OH is bonded immediately before the parent alcohol name. 4. Name each alkyl side chain (or other group), and designate ...
Student Learning Outcomes (broken down by chapter…basically the
... Write equations for acid-base reactions using curved arrows to show where the electrons start from and where they end up. Predict relative acidities and basicities. Predict how electronegativity, size, and hybridization affects acidity. Describe how substituents affect the strength of an acid. Chapt ...
... Write equations for acid-base reactions using curved arrows to show where the electrons start from and where they end up. Predict relative acidities and basicities. Predict how electronegativity, size, and hybridization affects acidity. Describe how substituents affect the strength of an acid. Chapt ...
Problem Set Chapter 10 Solutions January 30, 2013 10.17 Draw the
... But C11H34 has too many hydrogens to be a feasible molecular formula (c) A hydrocarbon with M+ = 84 C7 C6H12 C5H24 The only one of these formulas that will lead to a feasible structure is C6H12: ...
... But C11H34 has too many hydrogens to be a feasible molecular formula (c) A hydrocarbon with M+ = 84 C7 C6H12 C5H24 The only one of these formulas that will lead to a feasible structure is C6H12: ...
Ch 17- Aldehydes and Ketones
... have an -OH in the molecule, find the longest chain that contains both the carbon of the carbonyl and the carbon bonded to the -OH group • Number the chain to give the carbon of the carbonyl the lowest number • The -OH will be named as a substituent! • When the -OH group is named as a substituent, i ...
... have an -OH in the molecule, find the longest chain that contains both the carbon of the carbonyl and the carbon bonded to the -OH group • Number the chain to give the carbon of the carbonyl the lowest number • The -OH will be named as a substituent! • When the -OH group is named as a substituent, i ...
Carbon and its Compounds Summary Study of the compounds of
... Question (38): Write a short note on substitution reactions? Answer: The replacement of a hydrogen atom of a hydrocarbon molecule by an atom or a group of atoms is known as substitution reaction. Alkanes, due to their structure, can undergo substitution reactions only in the presence of sunlight or ...
... Question (38): Write a short note on substitution reactions? Answer: The replacement of a hydrogen atom of a hydrocarbon molecule by an atom or a group of atoms is known as substitution reaction. Alkanes, due to their structure, can undergo substitution reactions only in the presence of sunlight or ...
- Deans Community High School
... 13. Propan-1-ol, can be oxidised by passing the alcohol vapour over hot copper(II) oxide. a) Draw a labelled diagram of the apparatus that would be used to carry out this experiment in the aboratory. b) Oxidation of propan-1-ol yields a compound X, formula C3H6O, which can be further oxidised to co ...
... 13. Propan-1-ol, can be oxidised by passing the alcohol vapour over hot copper(II) oxide. a) Draw a labelled diagram of the apparatus that would be used to carry out this experiment in the aboratory. b) Oxidation of propan-1-ol yields a compound X, formula C3H6O, which can be further oxidised to co ...
Combi chemistry
... Synthetic methodologies for production of combinatorial libraries a) Solid phase synthesis the compound library have been synthesized on solid phase such as resin bead , pins or chips. b) solution phase synthesis In this method synthesis of compounds takes place in solution phase. ...
... Synthetic methodologies for production of combinatorial libraries a) Solid phase synthesis the compound library have been synthesized on solid phase such as resin bead , pins or chips. b) solution phase synthesis In this method synthesis of compounds takes place in solution phase. ...
UNIVERSITY OF CAMBRIDGE INTERNATIONAL
... Islay is an island off the west coast of Scotland. The main industry on the island is making ethanol from barley. Barley contains the complex carbohydrate, starch. Enzymes catalyse the hydrolysis of starch to a solution of glucose. (a) (i) Draw the structure of the starch. Glucose can be represented ...
... Islay is an island off the west coast of Scotland. The main industry on the island is making ethanol from barley. Barley contains the complex carbohydrate, starch. Enzymes catalyse the hydrolysis of starch to a solution of glucose. (a) (i) Draw the structure of the starch. Glucose can be represented ...
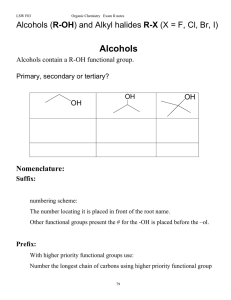
Alcohols (R-OH), and alkyl halides, RX
... ROH: Alkoxide: Alkoxides are important bases in organic chemistry. Replace the H with another R' group Æ Reaction with a carboxylic acid Æ Reaction with hydrogen halides Æ ...
... ROH: Alkoxide: Alkoxides are important bases in organic chemistry. Replace the H with another R' group Æ Reaction with a carboxylic acid Æ Reaction with hydrogen halides Æ ...
CHAPTER II. A Facile Synthesis of Arylacetic Acid Derivatives via
... Section B: A Novel Protocol for the Synthesis of β-Amino Carbonyl Compounds From Aza-Michael Reaction The aza-Michael reaction is an important reaction in organic chemistry especially for the synthesis of heterocycles, containing ,-amino carbonyl unit. ...
... Section B: A Novel Protocol for the Synthesis of β-Amino Carbonyl Compounds From Aza-Michael Reaction The aza-Michael reaction is an important reaction in organic chemistry especially for the synthesis of heterocycles, containing ,-amino carbonyl unit. ...
using hydrogen as a nucleophile in hydride reductions
... process is formation of the corresponding carbonyl compound (aldehyde or ketone), which may or may not undergo further reduction to alcohol, depending on the nature of the reagents used and reaction conditions. The following mechanism illustrates this concept. For simplicity, only the hydride ion is ...
... process is formation of the corresponding carbonyl compound (aldehyde or ketone), which may or may not undergo further reduction to alcohol, depending on the nature of the reagents used and reaction conditions. The following mechanism illustrates this concept. For simplicity, only the hydride ion is ...
Name - Clydebank High School
... c) Draw, the extended structural formula of the compound formed when bromine reacts with compound X. Name the compound formed. ...
... c) Draw, the extended structural formula of the compound formed when bromine reacts with compound X. Name the compound formed. ...
What is Organic Chemistry? - Westgate Mennonite Collegiate
... These highly reactive substances have many industrial uses. Again the naming of these compounds is similar to the alkanes except that the suffix is yne. ...
... These highly reactive substances have many industrial uses. Again the naming of these compounds is similar to the alkanes except that the suffix is yne. ...
program
... Domain C: Carbon chemistry Subdomain C2: Other applications of carbon bonds Subdomain C3: The candidate can indicate for a number of carbon bonds which types of reactions they can undergo and which products are then formed. The candidate can: ...
... Domain C: Carbon chemistry Subdomain C2: Other applications of carbon bonds Subdomain C3: The candidate can indicate for a number of carbon bonds which types of reactions they can undergo and which products are then formed. The candidate can: ...
BIOB111 - Tutorial activities for session 8
... Both straight chain (acyclic) and ring structured (cyclic) hydrocarbons can form structures that contain the same number of carbon atoms. However, these acyclic and cyclic compounds contain different numbers of hydrogen atoms, even though they have the same number of carbon atoms. Which of the follo ...
... Both straight chain (acyclic) and ring structured (cyclic) hydrocarbons can form structures that contain the same number of carbon atoms. However, these acyclic and cyclic compounds contain different numbers of hydrogen atoms, even though they have the same number of carbon atoms. Which of the follo ...
Aldehydes and Ketones
... If the CHO group is bonded to a ring, name the ring and add the suffix –carbaldehyde. Number the chain or ring to put the CHO group at C1, but omit this number from the name. Apply all the other usual rules of nomenclature. ...
... If the CHO group is bonded to a ring, name the ring and add the suffix –carbaldehyde. Number the chain or ring to put the CHO group at C1, but omit this number from the name. Apply all the other usual rules of nomenclature. ...
Article Summaries
... One of the major downsides to the original Shilov reaction is the fact that it uses platinum consumptively. The goal of this set of experiments was to determine if a non-consumptive pathway was possible. The scorpionate ligands Tp’ (hydrotris(3,5-dimethylpyrazolyl)borate) and Tp (hydridotris(pyrazol ...
... One of the major downsides to the original Shilov reaction is the fact that it uses platinum consumptively. The goal of this set of experiments was to determine if a non-consumptive pathway was possible. The scorpionate ligands Tp’ (hydrotris(3,5-dimethylpyrazolyl)borate) and Tp (hydridotris(pyrazol ...
Document
... Step 2: How many carbon atoms are in the branch (Not including the carbon that is a part of the carbon skeleton? ______ What is the prefix associated with that number? _________ Add the suffix –yl to the prefix from the previous question to make __________. Step 3: What would the name of the carbon ...
... Step 2: How many carbon atoms are in the branch (Not including the carbon that is a part of the carbon skeleton? ______ What is the prefix associated with that number? _________ Add the suffix –yl to the prefix from the previous question to make __________. Step 3: What would the name of the carbon ...
Chapter 9 Organic chemistry: The Infinite Varietyof Carbon
... – Show how many hydrogens are attached to each carbon CH3-CH3 for ethane ...
... – Show how many hydrogens are attached to each carbon CH3-CH3 for ethane ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.