Retrosynthesis - Organic Chemistry
... 6 Retrosynthesis : Summary of Reactions Do NOT start studying by trying to memorize the reactions here! Work as many problems as you can, with this list of reactions in front of you if necessary, so that you can get through as many problems as you can without getting stuck on eth reagents/condition ...
... 6 Retrosynthesis : Summary of Reactions Do NOT start studying by trying to memorize the reactions here! Work as many problems as you can, with this list of reactions in front of you if necessary, so that you can get through as many problems as you can without getting stuck on eth reagents/condition ...
Unit 4 Chemical Kinetics and Chemical Equilibrium
... Asymmetric reagents such as H-X add to a C=C so that the proton adds to the carbon (in the double bond) that already has the greater number of hydrogen atoms. “The rich get richer” ...
... Asymmetric reagents such as H-X add to a C=C so that the proton adds to the carbon (in the double bond) that already has the greater number of hydrogen atoms. “The rich get richer” ...
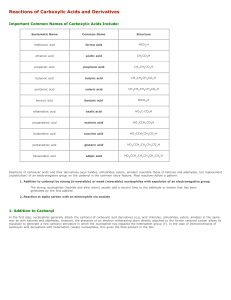
Reactions of Carboxylic Acids and Derivatives
... In the first step, nucleophiles generally attack the carbonyl of carboxylic acid derivatives (e.g. acid chlorides, anhydrides, esters, amides) in the same way as with ketones and aldehydes. However, the presence of an electron withdrawing atom directly attached to the former carbonyl carbon allows i ...
... In the first step, nucleophiles generally attack the carbonyl of carboxylic acid derivatives (e.g. acid chlorides, anhydrides, esters, amides) in the same way as with ketones and aldehydes. However, the presence of an electron withdrawing atom directly attached to the former carbonyl carbon allows i ...
Document
... Note that each time an ester group is formed a water molecule is lost. This type of polymer is known as a polyester, and this particular example, made from benzene-1,4-dicarboxylic acid (A) and ethane-1,2-diol (B), is marketed by ICI as Terylene®. Polyesters are suitable for fibres, and are widely u ...
... Note that each time an ester group is formed a water molecule is lost. This type of polymer is known as a polyester, and this particular example, made from benzene-1,4-dicarboxylic acid (A) and ethane-1,2-diol (B), is marketed by ICI as Terylene®. Polyesters are suitable for fibres, and are widely u ...
Biol 1020 Ch. 4: organic molecules
... carbon does not readily from ionic bonds it almost always forms covalent bonds ...
... carbon does not readily from ionic bonds it almost always forms covalent bonds ...
Atom Transfer Radical Polymerization and
... Of the methods developed based on this concept, one of the most useful is atom transfer radical addition (ATRA),[32,33] so named because it employs atom transfer from an organic halide to a transition-metal complex to generate the reacting radicals, followed by back transfer from the transition meta ...
... Of the methods developed based on this concept, one of the most useful is atom transfer radical addition (ATRA),[32,33] so named because it employs atom transfer from an organic halide to a transition-metal complex to generate the reacting radicals, followed by back transfer from the transition meta ...
Mass Spec - Fragmentation
... rules of Lewis structures (i.e. don’t put more than 8 electrons on carbon). ...
... rules of Lewis structures (i.e. don’t put more than 8 electrons on carbon). ...
Reactions of Molecules with Oxygen
... Oxidation Reactions of Alcohols What oxidizing agent is used in the selective oxidation of alcohols? Potassium dichromate, K2Cr2O7, is the oxidizing agent [O] used in many alcohol oxidation reactions. The K2Cr2O7 solution must be acidified first by adding sulfuric acid, H2SO4. This provides an appr ...
... Oxidation Reactions of Alcohols What oxidizing agent is used in the selective oxidation of alcohols? Potassium dichromate, K2Cr2O7, is the oxidizing agent [O] used in many alcohol oxidation reactions. The K2Cr2O7 solution must be acidified first by adding sulfuric acid, H2SO4. This provides an appr ...
Chapter 4 Alkanes
... Naming three- or four-carbon alkyl groups is more complicated because the parent hydrocarbons have more than one type of hydrogen atom. For example, propane has both 1° and 2° H atoms, and removal of each of these H atoms forms a different alkyl group with a different name, propyl or isopropyl. ...
... Naming three- or four-carbon alkyl groups is more complicated because the parent hydrocarbons have more than one type of hydrogen atom. For example, propane has both 1° and 2° H atoms, and removal of each of these H atoms forms a different alkyl group with a different name, propyl or isopropyl. ...
Lect 9 Alcohols
... • It is used in cosmetic surgery to remove layers of dead skin. It is also used in phenolization, a surgical procedure used to treat an ingrown nail, in which it is applied to the toe to prevent regrowth of nails. • 5% Phenol is sometimes injected near a sensory nerve in order to temporarily (up to ...
... • It is used in cosmetic surgery to remove layers of dead skin. It is also used in phenolization, a surgical procedure used to treat an ingrown nail, in which it is applied to the toe to prevent regrowth of nails. • 5% Phenol is sometimes injected near a sensory nerve in order to temporarily (up to ...
Carey Chapter 4 Alcohols, Alkyl Halides
... Number chain in direction that gives lowest number to the carbon that bears the —OH group. CH3CH2OH ...
... Number chain in direction that gives lowest number to the carbon that bears the —OH group. CH3CH2OH ...
Organic Chemistry
... This book was typeset in 10/12 New Baskerville at cMPreparé and printed and bound by Courier/Kendallville. The cover was printed by Courier/Kendallville. The paper in this book was manufactured by a mill whose forest management programs include sustained yield harvesting of its timberlands. Sustaine ...
... This book was typeset in 10/12 New Baskerville at cMPreparé and printed and bound by Courier/Kendallville. The cover was printed by Courier/Kendallville. The paper in this book was manufactured by a mill whose forest management programs include sustained yield harvesting of its timberlands. Sustaine ...
File
... Dehydration of Alcohols Alcohol Alkene + water • when heated with an acid catalyst:such as a concentrated acid (a dehydrating agent) or pumice, or Al2O3 • with the loss of H and OH to form water ...
... Dehydration of Alcohols Alcohol Alkene + water • when heated with an acid catalyst:such as a concentrated acid (a dehydrating agent) or pumice, or Al2O3 • with the loss of H and OH to form water ...
CH 320-328 M Synopsis
... • Be able to wrte the stepwise mechanisms for the electrophilic additions of HX, H2O/H3O , X2, and X2/H2O to carbon-carbon double bonds symbolizing the flow of electrons with curved arrows. • Know the stereochemical outcome of reactions such as the addition of X2 (anti), X2/H2O (or X2/CH3OH, etc.) ( ...
... • Be able to wrte the stepwise mechanisms for the electrophilic additions of HX, H2O/H3O , X2, and X2/H2O to carbon-carbon double bonds symbolizing the flow of electrons with curved arrows. • Know the stereochemical outcome of reactions such as the addition of X2 (anti), X2/H2O (or X2/CH3OH, etc.) ( ...
alcohols and oxidation products
... CH3COCH3 can be prepared in the laboratory from an alcohol. State the name of this alcohol, the type of reaction occurring and the reagents and conditions needed for the reaction. (Total 5 marks) ...
... CH3COCH3 can be prepared in the laboratory from an alcohol. State the name of this alcohol, the type of reaction occurring and the reagents and conditions needed for the reaction. (Total 5 marks) ...
Alcohols and Phenols
... • NaBH4 is not sensitive to moisture and it does not reduce other common functional groups • Lithium aluminum hydride (LiAlH4) is more powerful, less specific, and very reactive with water • Both add the equivalent of “H-” ...
... • NaBH4 is not sensitive to moisture and it does not reduce other common functional groups • Lithium aluminum hydride (LiAlH4) is more powerful, less specific, and very reactive with water • Both add the equivalent of “H-” ...
Chapter 7: Dienes
... Dienes & Polyenes: An overview and two key reactions (Ch. 14.1-14.5) Polyenes contain more than one double bond and are very common in natural products (ex: carotene). Diene chemistry applies to trienes, tetraenes, etc. The chemistry of polyenes depends on the relative positions of the C=C bonds: CH ...
... Dienes & Polyenes: An overview and two key reactions (Ch. 14.1-14.5) Polyenes contain more than one double bond and are very common in natural products (ex: carotene). Diene chemistry applies to trienes, tetraenes, etc. The chemistry of polyenes depends on the relative positions of the C=C bonds: CH ...
TOPIC 7. ELIMINATION REACTIONS (chapter 7 and parts of
... At the end of Topic 6 you were challenged to recognize one-step synthetic transformations. But not all transformations can be achieved in one step. For example, there is no method to dehydrogenate (i.e., remove H2) alkanes. So how would you bring about the following transformation? ...
... At the end of Topic 6 you were challenged to recognize one-step synthetic transformations. But not all transformations can be achieved in one step. For example, there is no method to dehydrogenate (i.e., remove H2) alkanes. So how would you bring about the following transformation? ...
Alcohols and Phenols
... • NaBH4 is not sensitive to moisture and it does not reduce other common functional groups • Lithium aluminum hydride (LiAlH4) is more powerful, less specific, and very reactive with water • Both add the equivalent of “H-” ...
... • NaBH4 is not sensitive to moisture and it does not reduce other common functional groups • Lithium aluminum hydride (LiAlH4) is more powerful, less specific, and very reactive with water • Both add the equivalent of “H-” ...
File
... hydrolysis of nitriles d. describe and carry out, where appropriate, the reactions of carboxylic acids. This will be limited to: i. ...
... hydrolysis of nitriles d. describe and carry out, where appropriate, the reactions of carboxylic acids. This will be limited to: i. ...
Alcohols and Phenols - faculty at Chemeketa
... substituents carbon come from the Grignard reagent Grignard reagents do not add to carboxylic acids – they undergo an acid-base reaction, generating the hydrocarbon of the Grignard reagent ...
... substituents carbon come from the Grignard reagent Grignard reagents do not add to carboxylic acids – they undergo an acid-base reaction, generating the hydrocarbon of the Grignard reagent ...
Carbonyl compounds
... electron rich oxygen can transfer its electron pair to a proton, thus completing the overall addition of Nu-H to the carbonyl group. The relative reactivities of aldehydes and ketones toward nucleophilic addition depend on two ...
... electron rich oxygen can transfer its electron pair to a proton, thus completing the overall addition of Nu-H to the carbonyl group. The relative reactivities of aldehydes and ketones toward nucleophilic addition depend on two ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.