Carbonyl Compounds notes
... alcohols; a carboxylic acid will give a sweet smell if heated with ethanol and a strong acid, and an alcohol will give a sweet smell if heated with ethanoic acid and a strong acid. Esters are also used as plasticizers. They can be added to polymers to make them less rigid and more flexible. Plastici ...
... alcohols; a carboxylic acid will give a sweet smell if heated with ethanol and a strong acid, and an alcohol will give a sweet smell if heated with ethanoic acid and a strong acid. Esters are also used as plasticizers. They can be added to polymers to make them less rigid and more flexible. Plastici ...
MECH 558 Combustion Class Notes
... If one were to measure all stable chemical species in a premixed flame, for example, it would look something like this: MECH 558 notes07.doc Combustion Hydrocarbon Combustion Chemistry Class Notes - Page: 2 Text: Ch. 5, App. 2A 2. Crash Course in Organic Chemistry Before learning how hydrocarbons re ...
... If one were to measure all stable chemical species in a premixed flame, for example, it would look something like this: MECH 558 notes07.doc Combustion Hydrocarbon Combustion Chemistry Class Notes - Page: 2 Text: Ch. 5, App. 2A 2. Crash Course in Organic Chemistry Before learning how hydrocarbons re ...
Tech Info - Davis Instruments
... Derivatives of the parent reactant molecules benzaldehyde and acetophenone can include a variety of R, R1 and R2 functional groups, such as methyl (-CH3), methoxy (-OCH3), chloro (-Cl), bromo (-Br), amino (-NH2), hydroxy (-OH), nitrile (-CN), etc. and in various positions around the phenyl ring. Due ...
... Derivatives of the parent reactant molecules benzaldehyde and acetophenone can include a variety of R, R1 and R2 functional groups, such as methyl (-CH3), methoxy (-OCH3), chloro (-Cl), bromo (-Br), amino (-NH2), hydroxy (-OH), nitrile (-CN), etc. and in various positions around the phenyl ring. Due ...
Document
... ● If the cpd. is insoluble in water, dissolve it in 1 mL of methanol & then add the reagent. 2- Differentiation between aldehydes and ketones: Differentiation between aldehydes and ketones is achieved by taking the advantage of the fact that aldehydes can be easily oxidized while ketones can not ( t ...
... ● If the cpd. is insoluble in water, dissolve it in 1 mL of methanol & then add the reagent. 2- Differentiation between aldehydes and ketones: Differentiation between aldehydes and ketones is achieved by taking the advantage of the fact that aldehydes can be easily oxidized while ketones can not ( t ...
The Fischer Indole Synthesis
... Substituents may be added anywhere on the above molecule to create an indole derivative. Several thousand indole derivatives appear annually in chemical literature.3 The Fischer indole synthesis is the most widely used and versatile method for indole synthesis. Reaction Mechanism: The Fischer indole ...
... Substituents may be added anywhere on the above molecule to create an indole derivative. Several thousand indole derivatives appear annually in chemical literature.3 The Fischer indole synthesis is the most widely used and versatile method for indole synthesis. Reaction Mechanism: The Fischer indole ...
Acidity of Alcohols
... The acidity increases as the negative charge at the OH decreases (delocalized): a) phenols are more acidic than Alcohols due to resonance effect (delocalization of the negative charge) ...
... The acidity increases as the negative charge at the OH decreases (delocalized): a) phenols are more acidic than Alcohols due to resonance effect (delocalization of the negative charge) ...
Chapter 22 and 23 Study Guide
... notes or help from anyone. You should review homework, quizzes and other assignments for complete test preparation. These questions are only examples. Different chemicals will be on the test. ...
... notes or help from anyone. You should review homework, quizzes and other assignments for complete test preparation. These questions are only examples. Different chemicals will be on the test. ...
examination paper - University of Calgary
... E bromine is a better leaving group than chlorine. ...
... E bromine is a better leaving group than chlorine. ...
Document
... One alkyl group is named as a hydrocarbon chain, and the other is named as part of a substituent bonded to that chain: Name the simpler alkyl group as an alkoxy substituent by changing the –yl ending of the alkyl group to –oxy. Name the remaining alkyl group as an alkane, with the alkoxy group as a ...
... One alkyl group is named as a hydrocarbon chain, and the other is named as part of a substituent bonded to that chain: Name the simpler alkyl group as an alkoxy substituent by changing the –yl ending of the alkyl group to –oxy. Name the remaining alkyl group as an alkane, with the alkoxy group as a ...
C - Deans Community High School
... a hint!!!! This is a primary alcohol because the OH group is attached to a carbon which is bonded to only one other carbon. H ...
... a hint!!!! This is a primary alcohol because the OH group is attached to a carbon which is bonded to only one other carbon. H ...
無投影片標題 - SKHSBS
... possible straight chain containing the C = C double bond and use the ending ‘-ene’ 2. Number the parent chain so as to include both carbon atoms of the double bond, and begin numbering with the end of the chain nearer the C = C double bond 3. Designate the position of the C = C double bond by using ...
... possible straight chain containing the C = C double bond and use the ending ‘-ene’ 2. Number the parent chain so as to include both carbon atoms of the double bond, and begin numbering with the end of the chain nearer the C = C double bond 3. Designate the position of the C = C double bond by using ...
Alcohols
... Relative Acidities of Alcohols and Steric Effects Alkyl groups make an alkoxide anion more difficult to be solvated and stabilized by water molecules and therefore make its formation less likely and the corresponding acid necessarily weaker The more easily the alkoxide ion is solvated by water th ...
... Relative Acidities of Alcohols and Steric Effects Alkyl groups make an alkoxide anion more difficult to be solvated and stabilized by water molecules and therefore make its formation less likely and the corresponding acid necessarily weaker The more easily the alkoxide ion is solvated by water th ...
Reductive Deoxygenation of Ketones and Secondary Alcohols by
... Friedel-Crafts alkylation products of the toluene by the (8) Structure 5, representing an aluminoxane dimer, is only exemplary of the wide variety of oligomers possibly formed. (9) (a) An AI-H bond either was initially present in the organoaluminum reagents employed or was potentially available from ...
... Friedel-Crafts alkylation products of the toluene by the (8) Structure 5, representing an aluminoxane dimer, is only exemplary of the wide variety of oligomers possibly formed. (9) (a) An AI-H bond either was initially present in the organoaluminum reagents employed or was potentially available from ...
Chapter 25 Alt Notes 0910
... Alcohols are considered neutral compounds because they are only very slightly acidic. Alcohols can behave as acids but only in the presence of very strong bases. ...
... Alcohols are considered neutral compounds because they are only very slightly acidic. Alcohols can behave as acids but only in the presence of very strong bases. ...
Adv_H_Unit_3_Pupil_N.. - Chemistry Teaching Resources
... Throughout the unit you will encounter and be expected to identify a variety of different types of reaction, some of which will be new to you. These will include: addition reduction ...
... Throughout the unit you will encounter and be expected to identify a variety of different types of reaction, some of which will be new to you. These will include: addition reduction ...
Naming of Alkanes
... Sources of Alkanes • Natural gas – 90 to 95 percent methane – 5 to 10 percent ethane, and – a mixture of other low-boiling alkanes, chiefly propane, butane, and 2methylpropane. • Petroleum – A thick liquid mixture of thousands of compounds, most of them hydrocarbons formed from the decomposition of ...
... Sources of Alkanes • Natural gas – 90 to 95 percent methane – 5 to 10 percent ethane, and – a mixture of other low-boiling alkanes, chiefly propane, butane, and 2methylpropane. • Petroleum – A thick liquid mixture of thousands of compounds, most of them hydrocarbons formed from the decomposition of ...
Slide 1
... This synthetic method allows for the selective preparation of primary amines with out the use of a large excess of amine. The synthesis involved the alkylation of the phthalimide anion, which is simply a protected ammonia that can only be alkylated once. ...
... This synthetic method allows for the selective preparation of primary amines with out the use of a large excess of amine. The synthesis involved the alkylation of the phthalimide anion, which is simply a protected ammonia that can only be alkylated once. ...
Organic Chemistry
... Michael Reaction, Cautions 1,4 vs 1,2 • Resonance-stabilized enolate anions and enamines are weak bases, react slowly with a,-unsaturated carbonyl compounds, and give 1,4-addition products. • Organolithium and Grignard reagents, on the other hand, are strong bases, add rapidly to carbonyl groups, ...
... Michael Reaction, Cautions 1,4 vs 1,2 • Resonance-stabilized enolate anions and enamines are weak bases, react slowly with a,-unsaturated carbonyl compounds, and give 1,4-addition products. • Organolithium and Grignard reagents, on the other hand, are strong bases, add rapidly to carbonyl groups, ...
Chapter 14 Solutions
... Ethanol is a polar molecule capable of hydrogen bonding with itself and water. Dimethyl ether is polar, but cannot hydrogen bond with itself. The stronger intermolecular forces make the boiling point of ethanol higher. Both ethanol and dimethyl ether have only two carbons and can hydrogen bond to H2 ...
... Ethanol is a polar molecule capable of hydrogen bonding with itself and water. Dimethyl ether is polar, but cannot hydrogen bond with itself. The stronger intermolecular forces make the boiling point of ethanol higher. Both ethanol and dimethyl ether have only two carbons and can hydrogen bond to H2 ...
MOLECULAR REPRESENTATIONS AND INFRARED
... b) (C2H5)2CHC≡C(CH2)3CH3 or CH3CH2CH(C2H5)C≡C(CH2)3CH3 d) (CH3)2CHCH2S(CH2)2CH(C2H5)C(CH3)3 ...
... b) (C2H5)2CHC≡C(CH2)3CH3 or CH3CH2CH(C2H5)C≡C(CH2)3CH3 d) (CH3)2CHCH2S(CH2)2CH(C2H5)C(CH3)3 ...
Extra Practice Problems for Sections 22.4-22.7
... 22.6 Conjugate Addition Reactions • More reactive nucleophiles (e.g. Grignard) are more likely to attack the carbonyl directly. WHY? • Enolates are generally less reactive than Grignards but more reactive than Gilman reagents, so enolates often give a mixture of 1,2- and 1,4- addition products • Do ...
... 22.6 Conjugate Addition Reactions • More reactive nucleophiles (e.g. Grignard) are more likely to attack the carbonyl directly. WHY? • Enolates are generally less reactive than Grignards but more reactive than Gilman reagents, so enolates often give a mixture of 1,2- and 1,4- addition products • Do ...
Chem 240 - Napa Valley College
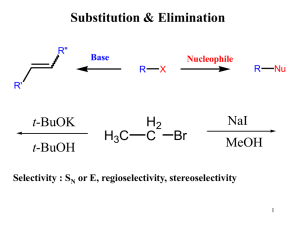
... mean that you would get a lot of by-products but you would end up getting more product also (SN1 major, E1 minor). 4) There are a number of ways of substituting a halogen for an alcohol group, but some ways are better than others. What advantage is there in using PCl3 rather than HCl in the chloride ...
... mean that you would get a lot of by-products but you would end up getting more product also (SN1 major, E1 minor). 4) There are a number of ways of substituting a halogen for an alcohol group, but some ways are better than others. What advantage is there in using PCl3 rather than HCl in the chloride ...
CHAPTER 8 Structures and nomenclature of organic
... unique structural and bonding properties. • The electronegativity of carbon is too low for a carbon atom to attract electrons from another element to form a negative ion, and it is too high to release electrons to another element to form a positive ion. Instead, it forms covalent bonds with many ot ...
... unique structural and bonding properties. • The electronegativity of carbon is too low for a carbon atom to attract electrons from another element to form a negative ion, and it is too high to release electrons to another element to form a positive ion. Instead, it forms covalent bonds with many ot ...
Chapter 23 - Simpson County Schools
... ketones that are water-soluble and easily dissolve in the bloodstream to be transported to tissues. At the same time, some of these ketones are reduced in the liver and the alcohol product is released in to the blood. ...
... ketones that are water-soluble and easily dissolve in the bloodstream to be transported to tissues. At the same time, some of these ketones are reduced in the liver and the alcohol product is released in to the blood. ...
Organic Chemistry Fifth Edition
... that elimination is the major reaction with all anionic nucleophiles. Only in solvolysis reactions does substitution predominate over elimination with tertiary alkyl halides. ...
... that elimination is the major reaction with all anionic nucleophiles. Only in solvolysis reactions does substitution predominate over elimination with tertiary alkyl halides. ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.