2013 Final Exam File - Fiji National University
... (c) Shown below is the structure of the dipeptide derivative aspartame, an artificial sweetener used in the beverage industry. ...
... (c) Shown below is the structure of the dipeptide derivative aspartame, an artificial sweetener used in the beverage industry. ...
3. Organic Compounds: Alkanes and
... Constitutional isomers are compounds that have the same chemical formula but different structures which differ in their connections between atoms © 2016 Cengage Learning. All Rights Reserved. ...
... Constitutional isomers are compounds that have the same chemical formula but different structures which differ in their connections between atoms © 2016 Cengage Learning. All Rights Reserved. ...
CH221 CLASS 13
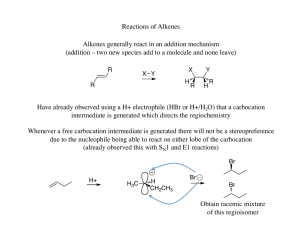
... Addition of water to alkenes to give alcohols is one of the most important reactions of alkenes. In industry, this is accomplished by the use of strong acid catalysts and high temperatures, but this is not really of much value in the laboratory. However, tertiary alcohols can be produced from highly ...
... Addition of water to alkenes to give alcohols is one of the most important reactions of alkenes. In industry, this is accomplished by the use of strong acid catalysts and high temperatures, but this is not really of much value in the laboratory. However, tertiary alcohols can be produced from highly ...
3.0 Properties of Phosgene
... azide can cause the formation of explosive carbazide. To prevent this reaction, completely remove excess phosgene; pass nitrogen into the solution prior to addition of the azide. Aluminum - Powdered aluminum burns in the vapor of phosgene. Alcohols - Phosgene reacts with all alcohols; two examples f ...
... azide can cause the formation of explosive carbazide. To prevent this reaction, completely remove excess phosgene; pass nitrogen into the solution prior to addition of the azide. Aluminum - Powdered aluminum burns in the vapor of phosgene. Alcohols - Phosgene reacts with all alcohols; two examples f ...
Organic Chemistry II
... Alkyl Halides We did talk about halo-alkanes (called alkyl halides) which are alkanes with a halogen attached. These molecules do, in fact, have polar bonds: C-Br, C-I, C-Cl are all polar bonds. Carbon is slightly positive, the halogen is slightly negative. ...
... Alkyl Halides We did talk about halo-alkanes (called alkyl halides) which are alkanes with a halogen attached. These molecules do, in fact, have polar bonds: C-Br, C-I, C-Cl are all polar bonds. Carbon is slightly positive, the halogen is slightly negative. ...
Ester-containing polyols having halogen and phosphorus atoms
... the daily use of other items. Numerous methods are known ...
... the daily use of other items. Numerous methods are known ...
Organic - UCLA Chemistry and Biochemistry
... whether one would obtain mainly the bromotetrahydropyrans or the bromotetrahydrofurans as the major products in this cyclization. Although the formation of the tetrahydropyrans is favored for mechanistic reasons (Markovnikov addition) and has been seen in some cases in the literature? there are also ...
... whether one would obtain mainly the bromotetrahydropyrans or the bromotetrahydrofurans as the major products in this cyclization. Although the formation of the tetrahydropyrans is favored for mechanistic reasons (Markovnikov addition) and has been seen in some cases in the literature? there are also ...
Organic Chemistry - City University of New York
... • If planar cyclopentadienyl cation were to exist, it would have 4 pi electrons and be antiaromatic. • Note that we can draw five equivalent contributing structures for the cyclopentadienyl cation. Yet this cation is not aromatic because it has only 4 pi ...
... • If planar cyclopentadienyl cation were to exist, it would have 4 pi electrons and be antiaromatic. • Note that we can draw five equivalent contributing structures for the cyclopentadienyl cation. Yet this cation is not aromatic because it has only 4 pi ...
Chapter 12: Oxidations In order to discuss the oxidation
... Oxidation of alkenes usually results in the loss of the pi bond And formation of two new sigma bonds between the carbons of the alkene and atoms more electronegative than carbon ...
... Oxidation of alkenes usually results in the loss of the pi bond And formation of two new sigma bonds between the carbons of the alkene and atoms more electronegative than carbon ...
CHAPTER 29 Organic chemicals
... (E) Halides of carboxylic acids are to be classified in the same heading as the corresponding acids. ...
... (E) Halides of carboxylic acids are to be classified in the same heading as the corresponding acids. ...
Reactions of Alkyl Halides (SN1, SN2, E1, and E2 reactions)
... dichloromethane solvent.) These oxidants are dissolved in cold anhydrous solvents. (no water– this weakens the oxidant.) 2. Moderate-to-strong oxidants are cold to warm, aqueous solutions of HNO3, acidic or basic KMnO4, NaOCl in aq. HAc, Jones reagent (CrO3 in aq. H2SO4 + acetone). 3. Severe oxidant ...
... dichloromethane solvent.) These oxidants are dissolved in cold anhydrous solvents. (no water– this weakens the oxidant.) 2. Moderate-to-strong oxidants are cold to warm, aqueous solutions of HNO3, acidic or basic KMnO4, NaOCl in aq. HAc, Jones reagent (CrO3 in aq. H2SO4 + acetone). 3. Severe oxidant ...
ch15[1].
... Characteristic Reactions • In the general reaction, we showed the nucleophile as an anion; this need not be the case. • Neutral molecules such as water, alcohols, ammonia, and amines can also serve as nucleophiles. • In the general reaction, we showed the leaving group as an anion to illustrate an ...
... Characteristic Reactions • In the general reaction, we showed the nucleophile as an anion; this need not be the case. • Neutral molecules such as water, alcohols, ammonia, and amines can also serve as nucleophiles. • In the general reaction, we showed the leaving group as an anion to illustrate an ...
fference: mechanistic How phenyl makes a di insights into the ruthenium( )-catalysed
... temperature, and represent the most accessible conditions for synthesis. However, the performance of the system was evaluated at lower catalyst loadings and higher temperatures in order to probe the limits of the catalyst. 1 can catalyse the reaction of 2, selectively producing 3 (77% conversion) at ...
... temperature, and represent the most accessible conditions for synthesis. However, the performance of the system was evaluated at lower catalyst loadings and higher temperatures in order to probe the limits of the catalyst. 1 can catalyse the reaction of 2, selectively producing 3 (77% conversion) at ...
Integration of chemical catalysis with extractive fermentation to
... Nearly one hundred years ago, the fermentative production of acetone by Clostridium acetobutylicum provided a crucial alternative source of this solvent for manufacture of the explosive cordite. Today there is a resurgence of interest in solventogenic Clostridium species to produce n-butanol and eth ...
... Nearly one hundred years ago, the fermentative production of acetone by Clostridium acetobutylicum provided a crucial alternative source of this solvent for manufacture of the explosive cordite. Today there is a resurgence of interest in solventogenic Clostridium species to produce n-butanol and eth ...
interaction of alcohols with alkalies under autogeneous pressure
... The apparatus consists of a high pressure bomb (1.35" i.d.; 3.65" 0.d.) made of molybdenum steel having a capacity of 380 c.c. (Fig. 4) tested to withstand pressures up to 1,000 atms. at 500° C. The bomb is placed in an electric furnace, the temperature of which can be controlled with a Sunvic regul ...
... The apparatus consists of a high pressure bomb (1.35" i.d.; 3.65" 0.d.) made of molybdenum steel having a capacity of 380 c.c. (Fig. 4) tested to withstand pressures up to 1,000 atms. at 500° C. The bomb is placed in an electric furnace, the temperature of which can be controlled with a Sunvic regul ...
Element Fact Sheet – Iodine
... Greek ἰοειδής ioeidēs, meaning violet or purple, due to the colour of elemental iodine vapour. Iodine and its compounds are primarily used in nutrition, and industrially in the production of acetic acid and certain polymers. Iodine's relatively high atomic number, low toxicity, and ease of attachmen ...
... Greek ἰοειδής ioeidēs, meaning violet or purple, due to the colour of elemental iodine vapour. Iodine and its compounds are primarily used in nutrition, and industrially in the production of acetic acid and certain polymers. Iodine's relatively high atomic number, low toxicity, and ease of attachmen ...
Mock Exam One
... a.) Reacting a ketone or an aldehyde with a primary amine will yield an imine. b.) Reacting a ketone or an aldehyde with a secondary amine will yield an enamine. c.) Imine and enamine formation should be carried out in highly acidic conditions. d.) Imines can be reduced to primary amines. e.) Enamin ...
... a.) Reacting a ketone or an aldehyde with a primary amine will yield an imine. b.) Reacting a ketone or an aldehyde with a secondary amine will yield an enamine. c.) Imine and enamine formation should be carried out in highly acidic conditions. d.) Imines can be reduced to primary amines. e.) Enamin ...
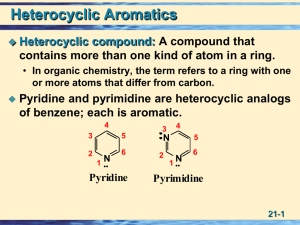
21 More About Amines • Heterocyclic Compounds
... Amines are also exceedingly important compounds to organic chemists, far too important to leave until the end of a course in organic chemistry. We have, therefore, already studied many aspects of amines and their chemistry. For example, we have seen that the nitrogen in amines is sp 3 hybridized and ...
... Amines are also exceedingly important compounds to organic chemists, far too important to leave until the end of a course in organic chemistry. We have, therefore, already studied many aspects of amines and their chemistry. For example, we have seen that the nitrogen in amines is sp 3 hybridized and ...
Chapter 11
... The electrophilic mercury reacts with an alkene to form a mercurinium ion which is similar to bromonium ions in that a three membered ring is formed with a partial bond to the carbon that can best handle the partial positive charge ...
... The electrophilic mercury reacts with an alkene to form a mercurinium ion which is similar to bromonium ions in that a three membered ring is formed with a partial bond to the carbon that can best handle the partial positive charge ...
Reactions of alcohols File
... -CH2OH -C-CH(OH)-Calcohols! NB Oxidant = hot acidified Cr2O72- [dichromate(VI)] ion] Provided by mixture of potassium dichromate(VI), K2Cr2O7, and excess dilute sulphuric acid, H2SO4. Oxidant is represented by : [O] ...
... -CH2OH -C-CH(OH)-Calcohols! NB Oxidant = hot acidified Cr2O72- [dichromate(VI)] ion] Provided by mixture of potassium dichromate(VI), K2Cr2O7, and excess dilute sulphuric acid, H2SO4. Oxidant is represented by : [O] ...
Alkenes - Gadjah Mada University
... CH3CH=CHCH3 + H2 CH3CH2CH2CH3 Hydrogenation requires high temperatures and pressures as well as the presence of a catalyst (e.g. Ni). ...
... CH3CH=CHCH3 + H2 CH3CH2CH2CH3 Hydrogenation requires high temperatures and pressures as well as the presence of a catalyst (e.g. Ni). ...
Topic 10.4 Organic Chemistry Alcohols
... If the oxygen in the –OH group is replaced with a sulfur atom, a group with somewhat similar properties results. The –SH functional group is known as a thiol or mercaptan. ...
... If the oxygen in the –OH group is replaced with a sulfur atom, a group with somewhat similar properties results. The –SH functional group is known as a thiol or mercaptan. ...
Rapid Ether and Alcohol CO Bond Hydrogenolysis Catalyzed by
... advantageous over previous protocols from this laboratory12,14 and others in many ways: (1) extended substrate scope including C−O bonds of alcohols and primary ethers; (2) high yields of alkanes without skeletal rearrangement; (3) commercially available catalysts with low metal loadings; (4) lower ...
... advantageous over previous protocols from this laboratory12,14 and others in many ways: (1) extended substrate scope including C−O bonds of alcohols and primary ethers; (2) high yields of alkanes without skeletal rearrangement; (3) commercially available catalysts with low metal loadings; (4) lower ...
Montmorillonite: An efficient, heterogeneous and
... Owing to approximately 0.9 to 1.2 nm of interlayer spacing and the excellent cation exchange property of MMT, MMT can form many nanocompsites with different organic compounds. Therefore, it expresses a significant capability to be used as a drug carrier for pharmaceutical purposes. Montmorillonite c ...
... Owing to approximately 0.9 to 1.2 nm of interlayer spacing and the excellent cation exchange property of MMT, MMT can form many nanocompsites with different organic compounds. Therefore, it expresses a significant capability to be used as a drug carrier for pharmaceutical purposes. Montmorillonite c ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.









![ch15[1].](http://s1.studyres.com/store/data/008194241_2-0a33cfb98ac502873dac865380b726e0-300x300.png)