Density Functional Study on the Preactivation Scenario of the
... complexes has not been carefully considered yet, despite the existence of similar reactions with other transition metals. For example, the reactions of (CO)5WCHC6H6 with alkenes were best explained by direct electrophilic attack of the carbene carbon at the alkene.25 Therefore, another possible mech ...
... complexes has not been carefully considered yet, despite the existence of similar reactions with other transition metals. For example, the reactions of (CO)5WCHC6H6 with alkenes were best explained by direct electrophilic attack of the carbene carbon at the alkene.25 Therefore, another possible mech ...
1. General introduction 1.1 Organometallic chemistry and homogeneous catalysis
... The success of organometallic catalysts lies in the easy modification of their environment by ligand exchange. A very large number of different types of ligands can coordinate to transition metal ions. Once the ligands are coordinated, the reactivity of the metals may change dramatically. In fact th ...
... The success of organometallic catalysts lies in the easy modification of their environment by ligand exchange. A very large number of different types of ligands can coordinate to transition metal ions. Once the ligands are coordinated, the reactivity of the metals may change dramatically. In fact th ...
History and Current Status of the Plastics Industry
... – Bonding of Carbon with 4 H, as in CH4, must satisfy 2 conditions • Each hydrogen must bond with one electron in the carbon cloud thus forming 4 bonds. This will satisfy octet rule for the p shell. ...
... – Bonding of Carbon with 4 H, as in CH4, must satisfy 2 conditions • Each hydrogen must bond with one electron in the carbon cloud thus forming 4 bonds. This will satisfy octet rule for the p shell. ...
Chapter 2 power point
... Covalent bonds form when elements share electrons, which usually occurs between nonmetals. ...
... Covalent bonds form when elements share electrons, which usually occurs between nonmetals. ...
Binuclear Metal Carbonyl DAB Complexes. III. Activation of a C=N
... = crdiimine) have been prepared and the electronic and spectroscopic properties investigated [l-6]. In almost all complexes the DAB ligand is u, acoordinated to the metal carbonyl fragment via the two lone pairs on nitrogen. Only one example has been reported where the nelectrons were involved in th ...
... = crdiimine) have been prepared and the electronic and spectroscopic properties investigated [l-6]. In almost all complexes the DAB ligand is u, acoordinated to the metal carbonyl fragment via the two lone pairs on nitrogen. Only one example has been reported where the nelectrons were involved in th ...
Types of Reactions and Solution Chemistry
... ability to react with each other. According to the Arrhenius theory, pure water dissociates to some extent to produce hydrogen ions, H+ and hydroxide ions, OH-. When this occurs, equal amounts of H+ and OH- ions are produced: H2O(l) H+(aq) + OH-(aq) An acid, according to Arrhenius, is any substanc ...
... ability to react with each other. According to the Arrhenius theory, pure water dissociates to some extent to produce hydrogen ions, H+ and hydroxide ions, OH-. When this occurs, equal amounts of H+ and OH- ions are produced: H2O(l) H+(aq) + OH-(aq) An acid, according to Arrhenius, is any substanc ...
Ligand effects on the X-ray absorption of a nickel
... of the XANES by 0.4 eV. All atoms out to 5.0 Angstroms were used in the matrix inversion of Eq. (14) to find the XANES. Using the X-ray diffraction geometry of the NiTPP(Pip)2 molecule, the XANES signatures of the ligands were investigated by selectively removing one or both ligands and repeating the ...
... of the XANES by 0.4 eV. All atoms out to 5.0 Angstroms were used in the matrix inversion of Eq. (14) to find the XANES. Using the X-ray diffraction geometry of the NiTPP(Pip)2 molecule, the XANES signatures of the ligands were investigated by selectively removing one or both ligands and repeating the ...
Question paper - Edexcel
... 10 When EDTA is added to [Cu(NH3)4]2+ in aqueous solution, the copper(II)-EDTA complex, [Cu(EDTA)]2–, predominates in the resulting solution. This is best explained by the fact that when [Cu(EDTA)]2– is formed from [Cu(NH3)4]2+ A there are much stronger bonds between the ligands and the copper(II) ...
... 10 When EDTA is added to [Cu(NH3)4]2+ in aqueous solution, the copper(II)-EDTA complex, [Cu(EDTA)]2–, predominates in the resulting solution. This is best explained by the fact that when [Cu(EDTA)]2– is formed from [Cu(NH3)4]2+ A there are much stronger bonds between the ligands and the copper(II) ...
Lecture 11 – Reaction Types and Mechanisms for Inorganic
... When M = Co(III) (low-spin d6), considerable crystal field stabilization is lost because the CFSE is so much greater for octahedral coordination than the CFSE than any possible geometry in 5- or 7-coordination. The loss in CFSE on going from 6-coordination to 5- or 7-coordination increases the activ ...
... When M = Co(III) (low-spin d6), considerable crystal field stabilization is lost because the CFSE is so much greater for octahedral coordination than the CFSE than any possible geometry in 5- or 7-coordination. The loss in CFSE on going from 6-coordination to 5- or 7-coordination increases the activ ...
The Journal Vol.92 (1-3)
... copper(II) complex employing the ligand hydrotris(3,5-diisopropyl-1-pyrazolyl)borate (HB(3,5iPr2pz)3) (see Figure 6). Similar complexes had only been described for f-block elements at that time. [64, 65] Preparation of the complex was performed at low temperatures either by the reaction of a monomer ...
... copper(II) complex employing the ligand hydrotris(3,5-diisopropyl-1-pyrazolyl)borate (HB(3,5iPr2pz)3) (see Figure 6). Similar complexes had only been described for f-block elements at that time. [64, 65] Preparation of the complex was performed at low temperatures either by the reaction of a monomer ...
Chemistry 30 Review of Basic Chemistry 20
... Compounds that dissolve in water may produce ions. These solutions are called electrolytes. Some compounds may dissolve in water but form no ions. These solutions are called nonelectrolytes. When electrolytes are formed, dissociation equations can be shown. ...
... Compounds that dissolve in water may produce ions. These solutions are called electrolytes. Some compounds may dissolve in water but form no ions. These solutions are called nonelectrolytes. When electrolytes are formed, dissociation equations can be shown. ...
Chem 115 POGIL Worksheet
... 3. Nonmetals tend to form anions with charges equal to their group number minus 8. Examples: F– (Group 7A, 7 - 8 = -1), O2– (Group 6A, 6 - 8 = -2), N3– (Group 5A, 5 - 8 = -3) ...
... 3. Nonmetals tend to form anions with charges equal to their group number minus 8. Examples: F– (Group 7A, 7 - 8 = -1), O2– (Group 6A, 6 - 8 = -2), N3– (Group 5A, 5 - 8 = -3) ...
transition metal complexes possessing tunable and switchable
... (R = Me, Ph, 4-AcPh) (see (iii) above) were used to synthesize four new complex salts c i s [RuII (NH 3 ) 4 (R2 Qpy2+)][PF6] 4 (R = Me 28, Ph 29, 4-AcPh 30, 2-Pym 31). The electronic absorption spectra of 17–31 display intense, visible MLCT bands, the energies of which decrease in the order R = Me > ...
... (R = Me, Ph, 4-AcPh) (see (iii) above) were used to synthesize four new complex salts c i s [RuII (NH 3 ) 4 (R2 Qpy2+)][PF6] 4 (R = Me 28, Ph 29, 4-AcPh 30, 2-Pym 31). The electronic absorption spectra of 17–31 display intense, visible MLCT bands, the energies of which decrease in the order R = Me > ...
Dalton Transactions PAPER Modeling the properties of lanthanoid single-ion magnets using an effective
... only for ionic metal complexes. When the nature of the ligand and the orientation of its electronic lone-pairs are taken into account, this simple picture is not realistic and the model needs to be improved. In this work we present an effective pointcharge model that intends to overcome this limitat ...
... only for ionic metal complexes. When the nature of the ligand and the orientation of its electronic lone-pairs are taken into account, this simple picture is not realistic and the model needs to be improved. In this work we present an effective pointcharge model that intends to overcome this limitat ...
Synthesis of chiral furanoside diphosphinite and thioether-phosphinite compounds derived from
... steps. The solution structures of the trigonal bipyramidal hydrodorhodium complexes with bidentate phosphorus ligand (P-P) [HRh(P-P)(CO)2], which are the resting states in the hydroformylation reaction, have been analyzed in detail.xi These complexes are generally assumed to have a trigonal bipyrami ...
... steps. The solution structures of the trigonal bipyramidal hydrodorhodium complexes with bidentate phosphorus ligand (P-P) [HRh(P-P)(CO)2], which are the resting states in the hydroformylation reaction, have been analyzed in detail.xi These complexes are generally assumed to have a trigonal bipyrami ...
Inorganic Mechanisms II Lecture Notes
... Understand distinction between outer sphere and inner sphere electron transfer. Describe how rearrangement of solvent and internal atom coordinates affects the rate of outer sphere electron transfer. Use the Marcus cross relation and discuss the relation ship between , G‡ and Go Understand that a ...
... Understand distinction between outer sphere and inner sphere electron transfer. Describe how rearrangement of solvent and internal atom coordinates affects the rate of outer sphere electron transfer. Use the Marcus cross relation and discuss the relation ship between , G‡ and Go Understand that a ...
1002_3rd Exam_1010523
... 24) Choose the INCORRECT statement about the electron configuration of transition elements. A) Zn: [Ar]3d 104s2 B) Cr: [Ar]3d 4 4s2 C) Fe: [Ar]3d 6 4s2 D) V: [Ar]3d 3 4s2 E) Cu: [Ar]3d 104s1 Answer: B 25) Choose the INCORRECT statement about transition elements. A) Coordination compounds are formed ...
... 24) Choose the INCORRECT statement about the electron configuration of transition elements. A) Zn: [Ar]3d 104s2 B) Cr: [Ar]3d 4 4s2 C) Fe: [Ar]3d 6 4s2 D) V: [Ar]3d 3 4s2 E) Cu: [Ar]3d 104s1 Answer: B 25) Choose the INCORRECT statement about transition elements. A) Coordination compounds are formed ...
CHM 211 - The Federal University of Agriculture, Abeokuta
... BONDING THEORIES; VALENCE BOND THEORY, MOLECULAR BOND THEORIES. ...
... BONDING THEORIES; VALENCE BOND THEORY, MOLECULAR BOND THEORIES. ...
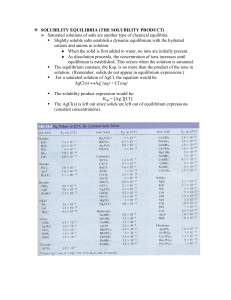
SOLUBILITY EQUILIBRIA
... When a metal ion (a Lewis acid) reacts with a Lewis base, a complex ion can form. The formation of complex ions represents a reversible equilibria situation. A complex ion is a charged species consisting of a metal ion surrounded by ligands. A ligand is typically an anion or neutral molecule ...
... When a metal ion (a Lewis acid) reacts with a Lewis base, a complex ion can form. The formation of complex ions represents a reversible equilibria situation. A complex ion is a charged species consisting of a metal ion surrounded by ligands. A ligand is typically an anion or neutral molecule ...
E5 Lewis Acids and Bases: Complexation
... Cu2 + exists as [Cu(H2O)4]2 + in aqueous solution. If 2 mL of 1.0 M NaOH is added to 2 mL of 0.10 M Cu(Cl)2, copper hydroxide precipitates. If HNO3(aq) is added to a portion of the precipitated sample, reaction occurs and the precipitate dissolves. If NH3(aq), is added to another portion of the prec ...
... Cu2 + exists as [Cu(H2O)4]2 + in aqueous solution. If 2 mL of 1.0 M NaOH is added to 2 mL of 0.10 M Cu(Cl)2, copper hydroxide precipitates. If HNO3(aq) is added to a portion of the precipitated sample, reaction occurs and the precipitate dissolves. If NH3(aq), is added to another portion of the prec ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.