08_chapter 1
... Borate glasses: Boron trioxide is a significant component of glasses, enamels and glazes. It is very rarely added to the raw material mixtures in the form of oxide, more frequent use being made of H3BO3 or Na2B4O7. Boron oxide (B2O3) usually occurs in the glassy form which is virtually incapable of ...
... Borate glasses: Boron trioxide is a significant component of glasses, enamels and glazes. It is very rarely added to the raw material mixtures in the form of oxide, more frequent use being made of H3BO3 or Na2B4O7. Boron oxide (B2O3) usually occurs in the glassy form which is virtually incapable of ...
NMR Shifts in Paramagnetic Systems: A Nonmultipole Expansion
... In this paper we take gS to be equal to 2 exactly. Here rN is the radius vector of the electron about the nucleus with nuclear spin angular momentum I, as shown in Fig. 1. The system we consider is a d’ system in a strong crystal field of octahedral symmetry, where bonding effects have been incorpor ...
... In this paper we take gS to be equal to 2 exactly. Here rN is the radius vector of the electron about the nucleus with nuclear spin angular momentum I, as shown in Fig. 1. The system we consider is a d’ system in a strong crystal field of octahedral symmetry, where bonding effects have been incorpor ...
Chemistry can be defined as the study of the composition, structure
... Phosphorous is one of the most abundant minerals in the human body, second only to calcium. This essential mineral is required for the healthy formation of bones and teeth, and is necessary for our bodies to process many of the foods that we eat. It is also a part of the body's energy storage system ...
... Phosphorous is one of the most abundant minerals in the human body, second only to calcium. This essential mineral is required for the healthy formation of bones and teeth, and is necessary for our bodies to process many of the foods that we eat. It is also a part of the body's energy storage system ...
Liquid chromatography: a tool for the analysis of metal species
... suitable for the columns selected. A low-capacity silica-based cation-exchange column was used for the separation of transition metals (Co, Cu, Fe) and coupled with post-column chemiluminescence detection to enhance sensitivity [39]. In this case attention has been paid to eluent composition not onl ...
... suitable for the columns selected. A low-capacity silica-based cation-exchange column was used for the separation of transition metals (Co, Cu, Fe) and coupled with post-column chemiluminescence detection to enhance sensitivity [39]. In this case attention has been paid to eluent composition not onl ...
Applications of UV
... have some of their d orbitals empty where a d-d transition can occur. The d-d transitions require excitation energy in the UV-Vis region. The direct interaction of the d electrons with ligands around the transition metal results in a spectrum of broad band nature. On the other hand, inner transition ...
... have some of their d orbitals empty where a d-d transition can occur. The d-d transitions require excitation energy in the UV-Vis region. The direct interaction of the d electrons with ligands around the transition metal results in a spectrum of broad band nature. On the other hand, inner transition ...
Chapter 07 and 08 Chemical Bonding and Molecular
... • Made of 2 or more elements in a definite proportion by mass • Physically and chemically different from the elements that make up the compound • All elements (except Noble gases) react to gain a stable octet. (duet-for H through B) • Compounds form to gain a stable valence shell which is LOWER IN E ...
... • Made of 2 or more elements in a definite proportion by mass • Physically and chemically different from the elements that make up the compound • All elements (except Noble gases) react to gain a stable octet. (duet-for H through B) • Compounds form to gain a stable valence shell which is LOWER IN E ...
Metal Chalcogenide Clusters with Closed Electronic Shells and the
... by CO. Furthermore, the PEt3 and CO derived states always reside either deeper or higher in energy relative to the Fermi level, respectively (Figure 5). Consequently, the progressive shift in the AIE and AEA is associated with the position of HOMO and LUMO states derived from the metal core rather t ...
... by CO. Furthermore, the PEt3 and CO derived states always reside either deeper or higher in energy relative to the Fermi level, respectively (Figure 5). Consequently, the progressive shift in the AIE and AEA is associated with the position of HOMO and LUMO states derived from the metal core rather t ...
Chemistry of Art by Ian Wilkinson
... end of the spectrum as smaller than at the low energy end In 1901, Max Plank developed the revolutionary quantum theory, which showed that light was emitted and absorbed in discrete units called photons, and that the energy of a photon was proportional to its frequency Rutherford’s model of the atom ...
... end of the spectrum as smaller than at the low energy end In 1901, Max Plank developed the revolutionary quantum theory, which showed that light was emitted and absorbed in discrete units called photons, and that the energy of a photon was proportional to its frequency Rutherford’s model of the atom ...
Answers to Self-Tests and Exercises
... Comparing the plots for 1s (Figure 1.10) and 2s (Figure 1.12) orbitals, the radial distribution function for a 1s orbital has a single maximum, and that for a 2s orbital has two maxima and a minimum (at r = 2ao/Z for hydrogenic 2s orbitals). The presence of the node at r = 2a0/Z for R(2s) requires t ...
... Comparing the plots for 1s (Figure 1.10) and 2s (Figure 1.12) orbitals, the radial distribution function for a 1s orbital has a single maximum, and that for a 2s orbital has two maxima and a minimum (at r = 2ao/Z for hydrogenic 2s orbitals). The presence of the node at r = 2a0/Z for R(2s) requires t ...
Iron-only hydrogenase: Synthetic, structural and reactivity studies of
... systems in biology. It is the -acid ligands, CO and CN, which undoubtedly allow access to these low oxidation/spinstate levels. Although stable higher oxidation state diiron units with CO/CN co-ligands remain elusive, Rauchfuss and co-workers [39] have shown that electron-rich Fe(II)–Fe(II) polyiso ...
... systems in biology. It is the -acid ligands, CO and CN, which undoubtedly allow access to these low oxidation/spinstate levels. Although stable higher oxidation state diiron units with CO/CN co-ligands remain elusive, Rauchfuss and co-workers [39] have shown that electron-rich Fe(II)–Fe(II) polyiso ...
Design of molecular structure and synthetic approaches
... SSP can be developed by applying the concept of geometrical molecular structure design, which is based on the choice of a type proper of molecular structure and its completion with ligands, providing the necessary number of donor atoms for the chosen core as well as sterical protection. Key words: p ...
... SSP can be developed by applying the concept of geometrical molecular structure design, which is based on the choice of a type proper of molecular structure and its completion with ligands, providing the necessary number of donor atoms for the chosen core as well as sterical protection. Key words: p ...
Final Review Answers
... 1) Define the following terms: (Look up) a) valence electrons b) octet rule c) malleable d) ductile 2) Differentiate between ionic, covalent, and metallic bonding in terms of electron location and types of atoms combined. Ionic - M/NM, e- donated; Covalent - NM, e- shared; Metallic - M, valence e- m ...
... 1) Define the following terms: (Look up) a) valence electrons b) octet rule c) malleable d) ductile 2) Differentiate between ionic, covalent, and metallic bonding in terms of electron location and types of atoms combined. Ionic - M/NM, e- donated; Covalent - NM, e- shared; Metallic - M, valence e- m ...
4. High molar extinction coefficient Ru(II)-mixed
... mLBD dyes were optimized in gaseous phase by using the density functional theory (DFT) with MPW1PW91 method and lanl2dz basis set for ruthenium and 6-31G(d) for H, C, N, O, S atoms as implemented in Gaussian 09W and Gaussian View 5 interface software. The unoccupied and occupied frontier molecular o ...
... mLBD dyes were optimized in gaseous phase by using the density functional theory (DFT) with MPW1PW91 method and lanl2dz basis set for ruthenium and 6-31G(d) for H, C, N, O, S atoms as implemented in Gaussian 09W and Gaussian View 5 interface software. The unoccupied and occupied frontier molecular o ...
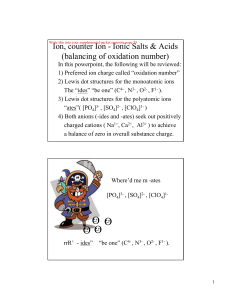
SrF 2(s)
... to go from name to formula: first part is the same as before…look up the symbol for each ion then balance the charges using subscripts, then for the hydrate part…add “ x H2O” where x is the number given in the prefix ...
... to go from name to formula: first part is the same as before…look up the symbol for each ion then balance the charges using subscripts, then for the hydrate part…add “ x H2O” where x is the number given in the prefix ...
Jahn-Teller assisted Na diffusion for high performance Na ion batteries
... whenever the Jahn−Teller active transition metal is facesharing with the activated site for sodium diffusion, including channeled31 and zigzag layered structures.32 A Jahn−Teller (JT) distortion lowers the energy of a system by certain bond length distortions to break the local crystal symmetry and l ...
... whenever the Jahn−Teller active transition metal is facesharing with the activated site for sodium diffusion, including channeled31 and zigzag layered structures.32 A Jahn−Teller (JT) distortion lowers the energy of a system by certain bond length distortions to break the local crystal symmetry and l ...
Metal-ligand bond lengths and strengths: Are they correlated? A
... this is probably a case of misplaced protons, as more logical hydrogen bonding is achieved with the longer Cu––O oxygen protonated giving the actual Cu––OHCOR as 2.521 Å, illustrating the problem of uncritically trusting the database. The longest Cu––O(H), 2.683 Å for catena-(bis(mD-Hydrogen malat ...
... this is probably a case of misplaced protons, as more logical hydrogen bonding is achieved with the longer Cu––O oxygen protonated giving the actual Cu––OHCOR as 2.521 Å, illustrating the problem of uncritically trusting the database. The longest Cu––O(H), 2.683 Å for catena-(bis(mD-Hydrogen malat ...
Acids and Bases The pH Scale
... is very close to 7.4, or slightly basic. A person cannot survive for more than a few minutes if the blood pH drops to 7 or rises to 7.8, and a chemical system exists in the blood that maintains a stable pH. If you add 0.01 mol of a strong acid to a liter of pure water, the pH drops from 7.0 to 2.0. ...
... is very close to 7.4, or slightly basic. A person cannot survive for more than a few minutes if the blood pH drops to 7 or rises to 7.8, and a chemical system exists in the blood that maintains a stable pH. If you add 0.01 mol of a strong acid to a liter of pure water, the pH drops from 7.0 to 2.0. ...
18-3-reading - WordPress.com
... atom of an element. The prefix is mono-. Many times monois not used. Instead, it is understood that if no prefix is used, there is only one atom of that element in a compound. In some cases, mono- is used for emphasis. Carbon monoxide is one example. ...
... atom of an element. The prefix is mono-. Many times monois not used. Instead, it is understood that if no prefix is used, there is only one atom of that element in a compound. In some cases, mono- is used for emphasis. Carbon monoxide is one example. ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.