Aqueous Reactions
... Aqueous Reactions Defining Aqueous Reactions Aqueous reactions are reactions that take place in water. To understand them, it is important to understand how compounds behave in water. Some compounds are electrolytes- they dissociate into separate ions in water. However, not all electrolytes behave t ...
... Aqueous Reactions Defining Aqueous Reactions Aqueous reactions are reactions that take place in water. To understand them, it is important to understand how compounds behave in water. Some compounds are electrolytes- they dissociate into separate ions in water. However, not all electrolytes behave t ...
Interaction of an extended series of N-substituted di(2
... architectures which in the case of [Cu(6)(ClO4 )2 (H2 O)]·0.5H2 O includes a chain-like structure formed by unusual intermolecular p-interactions between metal bound perchlorate anions and the aromatic rings of adjacent anthracenyl groups. Variable temperature magnetic susceptibility measurements ha ...
... architectures which in the case of [Cu(6)(ClO4 )2 (H2 O)]·0.5H2 O includes a chain-like structure formed by unusual intermolecular p-interactions between metal bound perchlorate anions and the aromatic rings of adjacent anthracenyl groups. Variable temperature magnetic susceptibility measurements ha ...
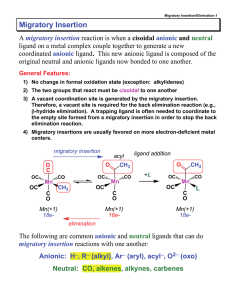
Migratory Insertion - vrg
... Normally this is a transition state structure for a hydride-alkene migratory insertion or a β-hydride elimination. In some cases, however, it can be observed as a ground-state stable structure. Because the C-H bond is sharing some of its σ-bond electron density with the metal, the C-H bond is weaken ...
... Normally this is a transition state structure for a hydride-alkene migratory insertion or a β-hydride elimination. In some cases, however, it can be observed as a ground-state stable structure. Because the C-H bond is sharing some of its σ-bond electron density with the metal, the C-H bond is weaken ...
The Rare Earth and Actinoid Elements
... (Table 24.1). Although most conventional designs of the periodic table show lutetium as a lanthanoid, its electron configuration as an element actually fits the pattern for the third transition series: [Xe]6s24f145dn (where n is 1, in this case). However, because all 15 elements from lanthanum to lu ...
... (Table 24.1). Although most conventional designs of the periodic table show lutetium as a lanthanoid, its electron configuration as an element actually fits the pattern for the third transition series: [Xe]6s24f145dn (where n is 1, in this case). However, because all 15 elements from lanthanum to lu ...
Chapter: The d and f Block Elements
... The actinoids are radioactive elements having relatively short half-lives. The elements of actinoid series could be prepared only in nanogram quantities. This is the reason why their study is more difficult. Question 5 What is mischmetall and what is its use? Ans. Mischmetall is an alloyof a lanthan ...
... The actinoids are radioactive elements having relatively short half-lives. The elements of actinoid series could be prepared only in nanogram quantities. This is the reason why their study is more difficult. Question 5 What is mischmetall and what is its use? Ans. Mischmetall is an alloyof a lanthan ...
ionization 12.3.1
... Multi-photon ionization It occurs when an atom or a molecule and their concomitant ions have energy states whereby the energy in two or more photons is absorbed. Negative ion chemical ionization See chemical ionization. Penning ionization Ionization occurs through the interaction of two or more neu ...
... Multi-photon ionization It occurs when an atom or a molecule and their concomitant ions have energy states whereby the energy in two or more photons is absorbed. Negative ion chemical ionization See chemical ionization. Penning ionization Ionization occurs through the interaction of two or more neu ...
WELCOME TO CLASS XII ORIENTATION IN CHEMISTRY SOME
... 473-523k and 35-36 bar pressure as shown in the equation belowAl2O3(s) + 2NaOH(aq) + 3H2O(l)-----2Na[Al(OH)4](aq) Alumina (Al2O3)is removed from the impure ore as sodium ...
... 473-523k and 35-36 bar pressure as shown in the equation belowAl2O3(s) + 2NaOH(aq) + 3H2O(l)-----2Na[Al(OH)4](aq) Alumina (Al2O3)is removed from the impure ore as sodium ...
d and f – Block Elements
... are absorbed. The transmitted ( unabsorbed) or reflected light ( or colour) appears coloured and gives the colour of compounds. The ions having no d-d transition are colourless. To understand the cause of colour in transition metal complexes, consider complex [Ti(H2O)6]3+ , In this case, titanium is ...
... are absorbed. The transmitted ( unabsorbed) or reflected light ( or colour) appears coloured and gives the colour of compounds. The ions having no d-d transition are colourless. To understand the cause of colour in transition metal complexes, consider complex [Ti(H2O)6]3+ , In this case, titanium is ...
Solubility and Complex-ion Equilibria
... • Many metal ions, especially transition metals, form coordinate covalent bonds with molecules or anions having a lone pair of electrons. – This type of bond formation is essentially a Lewis acid-base reaction (Chapter 16). – For example, the silver ion, Ag+, can react with ammonia to form the Ag(NH ...
... • Many metal ions, especially transition metals, form coordinate covalent bonds with molecules or anions having a lone pair of electrons. – This type of bond formation is essentially a Lewis acid-base reaction (Chapter 16). – For example, the silver ion, Ag+, can react with ammonia to form the Ag(NH ...
Small Reactive Sulfur-Nitrogen Compounds and Their Transition
... ~ ~ , ~ ~ development of the field was certainly hampered by the fact that the free ligand is not available under normal conditions, and indirect methods are required to build the thionitrosyl ligand within the coordination sphere, e.g., by sulfurization of nitrido-metal complexes or by halide abstr ...
... ~ ~ , ~ ~ development of the field was certainly hampered by the fact that the free ligand is not available under normal conditions, and indirect methods are required to build the thionitrosyl ligand within the coordination sphere, e.g., by sulfurization of nitrido-metal complexes or by halide abstr ...
Good Example of Report 3
... and has been shown to dramatically increase healing time. Unlike insulin, however, vanadium(IV) acetylacetonate will not cause hypoglycemia.2 In addition to having a variety of applications, vanadyl acetylacetonate and other metal chelate complexes are also interesting to study due to their unique c ...
... and has been shown to dramatically increase healing time. Unlike insulin, however, vanadium(IV) acetylacetonate will not cause hypoglycemia.2 In addition to having a variety of applications, vanadyl acetylacetonate and other metal chelate complexes are also interesting to study due to their unique c ...
Oxidation-Reduction Reactions
... Oxidation-reduction reactions, or redox reactions, are technically defined as any chemical reaction in which the oxidation number of the participating atom, ion, or molecule of a chemical compound changes. Some common redox reactions include fire, rusting of metals, browning of fruit, and photosynth ...
... Oxidation-reduction reactions, or redox reactions, are technically defined as any chemical reaction in which the oxidation number of the participating atom, ion, or molecule of a chemical compound changes. Some common redox reactions include fire, rusting of metals, browning of fruit, and photosynth ...
Mechanisms of Oxidation with Oxygen
... alternative ways of arranging the two electrons in the 2pTr(a) orbitals will be considered later. T h e y lead to higher energies because electron-electron repulsions are greater for them, and they correspond to excited states of the oxygen molecule. T h e excess of bonding over antibonding electron ...
... alternative ways of arranging the two electrons in the 2pTr(a) orbitals will be considered later. T h e y lead to higher energies because electron-electron repulsions are greater for them, and they correspond to excited states of the oxygen molecule. T h e excess of bonding over antibonding electron ...
4.6 Oxidation-Reduction (Redox) Reactions Oxidation Reduction
... Identifying Redox Reactions First determine oxidation numbers of each species in the reaction and then identify the oxidation and reduction processes A. Oxidation and reduction occur together. Whenever an atom loses electrons (is oxidized) another atom must gain those electrons (be reduced). B. Redu ...
... Identifying Redox Reactions First determine oxidation numbers of each species in the reaction and then identify the oxidation and reduction processes A. Oxidation and reduction occur together. Whenever an atom loses electrons (is oxidized) another atom must gain those electrons (be reduced). B. Redu ...
expansion and electrical conductivity of montmorillonite in ammonia
... NHg, small bands appear at about 2860 and 3070 c m - i . This may be attributed to N H stretch bands of the NH4+ ion, which is perturbed or affected b y the NHg. As the NH3 is removed, this band always disappears and the unperturbed N H stretching of NH4+ ion at 3280 cm-^ is enhanced. When the films ...
... NHg, small bands appear at about 2860 and 3070 c m - i . This may be attributed to N H stretch bands of the NH4+ ion, which is perturbed or affected b y the NHg. As the NH3 is removed, this band always disappears and the unperturbed N H stretching of NH4+ ion at 3280 cm-^ is enhanced. When the films ...
Redox Reactions: Electron Transfer
... Both the starting Co3+ (d6) and product Cr3+ (d3) chloride complexes are substitutionally inert so Cl- transfer must have occurred via a bridge species. ...
... Both the starting Co3+ (d6) and product Cr3+ (d3) chloride complexes are substitutionally inert so Cl- transfer must have occurred via a bridge species. ...
The Chemical Bond John Murrell Early Ideas Atoms come together
... We can use Lewis theory to understand some features of molecular structure if we analyse the consequences of electrostatic repulsion. We start by adopting the principle that electron pairing is an important feature of the chemical bond, and count the number of electron pairs around an atom. Consider ...
... We can use Lewis theory to understand some features of molecular structure if we analyse the consequences of electrostatic repulsion. We start by adopting the principle that electron pairing is an important feature of the chemical bond, and count the number of electron pairs around an atom. Consider ...
mod-5-revision-guide-4-transition-metals
... Insufficient volumes of sulphuric acid will mean the solution is not acidic enough and MnO2 will be produced instead of Mn2+. MnO4-(aq) + 4H+(aq) + 3e- MnO2 (s) + 2H2O The brown MnO2 will mask the colour change and lead to a greater (inaccurate) volume of Manganate being used in the titration. Usi ...
... Insufficient volumes of sulphuric acid will mean the solution is not acidic enough and MnO2 will be produced instead of Mn2+. MnO4-(aq) + 4H+(aq) + 3e- MnO2 (s) + 2H2O The brown MnO2 will mask the colour change and lead to a greater (inaccurate) volume of Manganate being used in the titration. Usi ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.