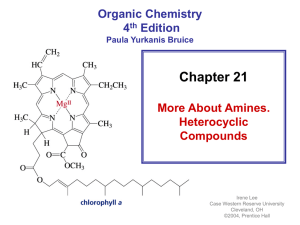
A fluoro-bridged dinuclear nickel(II) compound from
... Fig. 1 UV-vis spectrum of complex (1) (curve 1) and the (L) (curve 2) in CH2Cl2. ...
... Fig. 1 UV-vis spectrum of complex (1) (curve 1) and the (L) (curve 2) in CH2Cl2. ...
Lecture Resource ()
... The resonance hybrid of pyrrole indicates that there is a partial positive charge on the nitrogen ...
... The resonance hybrid of pyrrole indicates that there is a partial positive charge on the nitrogen ...
The Alkaline Earth Metals (Group 2) - Chemwiki
... significantly greater than that of the alkali metal immediately preceding it. The group 2 elements do exhibit some anomalies, however. For example, the density of Ca is less than that of Be and Mg, the two lightest members of the group, and Mg has the lowest melting and boiling points. In contrast t ...
... significantly greater than that of the alkali metal immediately preceding it. The group 2 elements do exhibit some anomalies, however. For example, the density of Ca is less than that of Be and Mg, the two lightest members of the group, and Mg has the lowest melting and boiling points. In contrast t ...
Problem 1 - eko.olunet.org
... acid, two modified peptides, A′ and B′ which have net charges +5 and –3 respectively are produced at pH 7.0. Calculate the net charge of the original peptide at the same pH. b) When peptide C (MW 464.5) is completely hydrolysed by aqueous HCl, equimolar quantities of glycine (Gly), phenylalanine (Ph ...
... acid, two modified peptides, A′ and B′ which have net charges +5 and –3 respectively are produced at pH 7.0. Calculate the net charge of the original peptide at the same pH. b) When peptide C (MW 464.5) is completely hydrolysed by aqueous HCl, equimolar quantities of glycine (Gly), phenylalanine (Ph ...
Chemistry 5325/5326 Inorganic Chemistry Spring Semester 2012
... Light absorption promotes an electron from the metal d orbitals to the Ligand π ∗ orbitals, dπ - π ∗ A number of electric-dipole-allowed charge-transfer transitions are observed which give rise to intense absorption bands in the visible region with moderate extinction coefficients. There is no forma ...
... Light absorption promotes an electron from the metal d orbitals to the Ligand π ∗ orbitals, dπ - π ∗ A number of electric-dipole-allowed charge-transfer transitions are observed which give rise to intense absorption bands in the visible region with moderate extinction coefficients. There is no forma ...
Worksheet to accompany demos on exchange reactions
... Example 1: If Al becomes Al3+ during some chemical change, we say that Al was oxidized. Electrons were taken from it and it became more positive. Example 2: If Cl- ions are converted into Cl2 molecules then we say that Cl- (or simply ‖Cl‖) was oxidized. Each Cl "atom" started off having a NEGATIVE c ...
... Example 1: If Al becomes Al3+ during some chemical change, we say that Al was oxidized. Electrons were taken from it and it became more positive. Example 2: If Cl- ions are converted into Cl2 molecules then we say that Cl- (or simply ‖Cl‖) was oxidized. Each Cl "atom" started off having a NEGATIVE c ...
Voltammetric characterisation of the self-assembled monolayers (SAMs) of benzyl- and dodecyl-mercapto
... DCM. Well established voltammetric methods [29] and [30] were used to investigate the integrity of the SAMs formed on gold electrodes. An investigation of the minimum time needed for the formation of a stable and well packed SAM was conducted with the use of the cyclic voltammetry curves of [Fe(CN)6 ...
... DCM. Well established voltammetric methods [29] and [30] were used to investigate the integrity of the SAMs formed on gold electrodes. An investigation of the minimum time needed for the formation of a stable and well packed SAM was conducted with the use of the cyclic voltammetry curves of [Fe(CN)6 ...
Condition - Future Website of mrbentley2
... 6. Draw the Lewis dot structures of the following ionic compounds. Then, using a different colored pen, show how one element “steals” the other’s electrons, resulting in two ions. (Hint: Some of the compounds may require multiple numbers of one type of element - be sure to draw in the extra element ...
... 6. Draw the Lewis dot structures of the following ionic compounds. Then, using a different colored pen, show how one element “steals” the other’s electrons, resulting in two ions. (Hint: Some of the compounds may require multiple numbers of one type of element - be sure to draw in the extra element ...
CC 15 1590-1591..4554k chapter .. Page1590
... expected bisignate CD curve upon complexation with tweezer 2 (see ESI†); but with other substrates, carrier 3 gave weak CD couplets with inconsistent signs. Although oxygen nucleophiles are weaker axial ligands of Zn porphyrins than nitrogen ones,10 it is known that the coordination between neutral ...
... expected bisignate CD curve upon complexation with tweezer 2 (see ESI†); but with other substrates, carrier 3 gave weak CD couplets with inconsistent signs. Although oxygen nucleophiles are weaker axial ligands of Zn porphyrins than nitrogen ones,10 it is known that the coordination between neutral ...
Monocopper Doping in Cd-In-S Supertetrahedral Nanocluster via
... the doping chemistry at different sites. For a T4-ZnGaSe cluster with 20 metal sites and 35 nonmetal sites, we successfully realized the site-selective and ordered distribution of two types of dopants (S and Sn) in a pentanary T4-ZnGaSnSeS cluster via synergistic effects of hard/soft acid/base theory ...
... the doping chemistry at different sites. For a T4-ZnGaSe cluster with 20 metal sites and 35 nonmetal sites, we successfully realized the site-selective and ordered distribution of two types of dopants (S and Sn) in a pentanary T4-ZnGaSnSeS cluster via synergistic effects of hard/soft acid/base theory ...
Deans Community High School Intermediate 2 Revision Notes www
... reactants, and the more energy that these collision have, the more likely it will be that these collisions are successful and cause a chemical reaction to take place. When chemical processes are performed on a large scale in industry, the costs can be extremely high if the reactions require a large ...
... reactants, and the more energy that these collision have, the more likely it will be that these collisions are successful and cause a chemical reaction to take place. When chemical processes are performed on a large scale in industry, the costs can be extremely high if the reactions require a large ...
Second-Sphere Contributions to Substrate-Analogue
... nature of the yellow and pink low-temperature azide adducts of iron(III) superoxide dismutase (N3-FeSOD), which have been known for more than two decades. Variable-temperature variable-field magnetic circular dichroism (MCD) data suggest that both species possess similar ferric centers with a single ...
... nature of the yellow and pink low-temperature azide adducts of iron(III) superoxide dismutase (N3-FeSOD), which have been known for more than two decades. Variable-temperature variable-field magnetic circular dichroism (MCD) data suggest that both species possess similar ferric centers with a single ...
Determination of the Copper Content in a Copper Clad Penny 2
... Complex ions are ions formed by the bonding of a metal atom or ion to two or more ligands by coordinate covalent bonds. A ligand is a negative ion or neutral molecule attached to the central metal ion in a complex ion. Many of these species are highly colored due to their ability to absorb light in ...
... Complex ions are ions formed by the bonding of a metal atom or ion to two or more ligands by coordinate covalent bonds. A ligand is a negative ion or neutral molecule attached to the central metal ion in a complex ion. Many of these species are highly colored due to their ability to absorb light in ...
REDOX EQUILIBRIA SL - chemistryatdulwich
... State the names of compounds using oxidation numbers. Deduce whether an element undergoes oxidation or reduction in reactions using oxidation numbers. ...
... State the names of compounds using oxidation numbers. Deduce whether an element undergoes oxidation or reduction in reactions using oxidation numbers. ...
REDOX EQUILIBRIA SL - chemistryatdulwich
... State the names of compounds using oxidation numbers. Deduce whether an element undergoes oxidation or reduction in reactions using oxidation numbers. ...
... State the names of compounds using oxidation numbers. Deduce whether an element undergoes oxidation or reduction in reactions using oxidation numbers. ...
REDOX EQUILIBRIA SL - chemistryatdulwich
... State the names of compounds using oxidation numbers. Deduce whether an element undergoes oxidation or reduction in reactions using oxidation numbers. ...
... State the names of compounds using oxidation numbers. Deduce whether an element undergoes oxidation or reduction in reactions using oxidation numbers. ...
LIQUIDS
... In a solid the particles are held in position by bonding to their neighbours. (A on the graph). As the solid is warmed the particles vibrate but cannot move. (Between A and B on the graph). When heated enough the particles vibrate so much that they can tear themselves free from their positions, and ...
... In a solid the particles are held in position by bonding to their neighbours. (A on the graph). As the solid is warmed the particles vibrate but cannot move. (Between A and B on the graph). When heated enough the particles vibrate so much that they can tear themselves free from their positions, and ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.