Scientific abstract
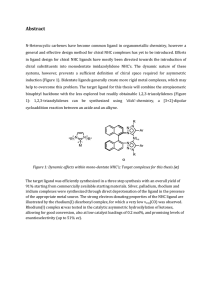
... 1): 1,2,3-triazolylidenes can be synthesized using ‘click’-chemistry, a [3+2]-dipolar cycloaddition reaction between an azide and an alkyne. ...
... 1): 1,2,3-triazolylidenes can be synthesized using ‘click’-chemistry, a [3+2]-dipolar cycloaddition reaction between an azide and an alkyne. ...
Dr. Stuart Batten
... ligands and complexes that will produce such higher assemblies which retain chemically active sites (e.g. coordination sites on metal atoms which are available for further chemical reactions; uncoordinated ligand donor atoms) after assembly. Such materials may be useful for homogeneous or heterogene ...
... ligands and complexes that will produce such higher assemblies which retain chemically active sites (e.g. coordination sites on metal atoms which are available for further chemical reactions; uncoordinated ligand donor atoms) after assembly. Such materials may be useful for homogeneous or heterogene ...
Ultra rigid cross-bridged tetraazamacrocycles as ligands—the
... ion and the Nax–M–Nax bond angle, which increases smoothly from MnII through CuII as the smaller metal ions can more easily be engulfed by the macrobicycle. MnII(5)Cl2 (Fig. 1) exemplifies these structures,§ and in this example the N(3)–Mn(1)–N(4) angle is 158.0°. Because of their great importance i ...
... ion and the Nax–M–Nax bond angle, which increases smoothly from MnII through CuII as the smaller metal ions can more easily be engulfed by the macrobicycle. MnII(5)Cl2 (Fig. 1) exemplifies these structures,§ and in this example the N(3)–Mn(1)–N(4) angle is 158.0°. Because of their great importance i ...
TRANSITION METAL CHEMISTRY –PART 3 –class notes
... 4. Which of the following octahedral complexes should have the largest crystal field splitting energy? A) [Cr(NH3]3+ B) [CrF6]3- C) [Cr(H2O)]3+ D) [Cr(CN)6]3- E) [CrCl6]35. CN- is a strong field ligand, whereas Cl- is usually a weak field ligand. Which of the following octahedral complexes has no un ...
... 4. Which of the following octahedral complexes should have the largest crystal field splitting energy? A) [Cr(NH3]3+ B) [CrF6]3- C) [Cr(H2O)]3+ D) [Cr(CN)6]3- E) [CrCl6]35. CN- is a strong field ligand, whereas Cl- is usually a weak field ligand. Which of the following octahedral complexes has no un ...
Ligand field theory
... The ordering of the ligands in their ability to cause d-orbital splitting. I- < Br- < Cl- < SCN- < NO3- < OH- < C2O42- < H2O ~ RS- < NCS- < NH3 ~ imidazole < en < bipy < phen < NO2- < PPh3 < CN- < CO Variations are due to σ-donating and Π-accepting properties of the ligand. Small Δ-splitting ligands ...
... The ordering of the ligands in their ability to cause d-orbital splitting. I- < Br- < Cl- < SCN- < NO3- < OH- < C2O42- < H2O ~ RS- < NCS- < NH3 ~ imidazole < en < bipy < phen < NO2- < PPh3 < CN- < CO Variations are due to σ-donating and Π-accepting properties of the ligand. Small Δ-splitting ligands ...
Complex Ions and Free Energy
... form between metal ions and ligands. Furthermore, I can determine the coordination number for a coordination complex • LT 8.7 – I can calculate the formation constant for complex ions and relate that to the Ksp for a slightly soluble compound. • LT 8.8 – I can calculate the free energy of a chemical ...
... form between metal ions and ligands. Furthermore, I can determine the coordination number for a coordination complex • LT 8.7 – I can calculate the formation constant for complex ions and relate that to the Ksp for a slightly soluble compound. • LT 8.8 – I can calculate the free energy of a chemical ...
1 5.03, Inorganic Chemistry Prof. Daniel G. Nocera Lecture 27 April 11
... Also, as the charge the metal ion increases, the metal becomes more electronegative, and hence the electronegativity difference between the metal and ligand atomic orbitals decreases owing to stabilization of EM relative to EL. Because ∆EML decreases, the interaction energy based on the Wolfsberg– H ...
... Also, as the charge the metal ion increases, the metal becomes more electronegative, and hence the electronegativity difference between the metal and ligand atomic orbitals decreases owing to stabilization of EM relative to EL. Because ∆EML decreases, the interaction energy based on the Wolfsberg– H ...
Transition Elements/Coordination Chemistry
... Many colorful compounds have transition metals. Both ionic and [complex coordination] compounds can be formed The properties of the transition metals are similar to each other and very different to the properties of the main group metals high mp, high densities, moderate to very hard, very good elec ...
... Many colorful compounds have transition metals. Both ionic and [complex coordination] compounds can be formed The properties of the transition metals are similar to each other and very different to the properties of the main group metals high mp, high densities, moderate to very hard, very good elec ...
Problem set 1
... The bulky, rigid tridentate ligand NNN is a good ligand for Ln3+ ions. Its rigidity means that it always coordinates in such a way as to occupy a triangular face: ...
... The bulky, rigid tridentate ligand NNN is a good ligand for Ln3+ ions. Its rigidity means that it always coordinates in such a way as to occupy a triangular face: ...
CHEM 415
... Activity 5B: Ligand Field Model (continued): Applications for Metal Complexes Background The properties of metal complexes can be understood from the energy splitting of the metal ion’s dorbitals in the lower symmetry that the ligands impose. This feature combined with the number of d electrons of t ...
... Activity 5B: Ligand Field Model (continued): Applications for Metal Complexes Background The properties of metal complexes can be understood from the energy splitting of the metal ion’s dorbitals in the lower symmetry that the ligands impose. This feature combined with the number of d electrons of t ...
Cu(NH3)4 - Granite Bay High School / Granite Bay High School
... (8) chelates are complexes formed by polydentate ligands; "chelate" comes from Greek for crab's claw (9) complexes used in sensitive qualitative tests to detect presence of ions in parts per million (ppm) range (10) hydrated ionic salts have water molecules as ligands and are thus complex ions/coord ...
... (8) chelates are complexes formed by polydentate ligands; "chelate" comes from Greek for crab's claw (9) complexes used in sensitive qualitative tests to detect presence of ions in parts per million (ppm) range (10) hydrated ionic salts have water molecules as ligands and are thus complex ions/coord ...
1.1 Werner`s Coordination Theory 1.2 Coordination
... When naming the metal complex, first name the ligands, in alphabetical order, followed f by the name off the metal a. If ligand is an anion whose name ends in –ite or –ate, the final “e” is change to an “o” ...
... When naming the metal complex, first name the ligands, in alphabetical order, followed f by the name off the metal a. If ligand is an anion whose name ends in –ite or –ate, the final “e” is change to an “o” ...
Ch 22 Transition complexes
... 7. A complex ion is a charged species consisting of a metal ion surrounded by [A] hydrogen ions. [B] ligands. [C] ligands and counter ions. [D] other transition metals. [E] none of these 8. What is the electron configuration of the Mn(II) ion? [A] [Ar] 4s23d3 [B] [Ar] 3d5 [C] [Ar] 4s23d5 [D] [Ar] 4s ...
... 7. A complex ion is a charged species consisting of a metal ion surrounded by [A] hydrogen ions. [B] ligands. [C] ligands and counter ions. [D] other transition metals. [E] none of these 8. What is the electron configuration of the Mn(II) ion? [A] [Ar] 4s23d3 [B] [Ar] 3d5 [C] [Ar] 4s23d5 [D] [Ar] 4s ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... What is intensity stealing? Mention its consequences. The CN stretching vibrations in cyano complexes occur at higher energy than that of free cyanide ion. Explain. Metal carbonyls are formed by metals in their lower oxidation states. Give reason. What are optically transparent electrodes? Mention t ...
... What is intensity stealing? Mention its consequences. The CN stretching vibrations in cyano complexes occur at higher energy than that of free cyanide ion. Explain. Metal carbonyls are formed by metals in their lower oxidation states. Give reason. What are optically transparent electrodes? Mention t ...
Staff demonstrating hours for level-3 Inorganic Lab
... W(=CH2) 1.94 Å Difficult to separate effects of 3 components in metal complexes. Evidence for -donor orbital (cases where no -bonding is possible) Lewis adduct H3BCO. Complex has (CO) at 2164cm-1 , free CO at 2143cm-1 therefore C-O bond order is increased with donation, as predicted. Same f ...
... W(=CH2) 1.94 Å Difficult to separate effects of 3 components in metal complexes. Evidence for -donor orbital (cases where no -bonding is possible) Lewis adduct H3BCO. Complex has (CO) at 2164cm-1 , free CO at 2143cm-1 therefore C-O bond order is increased with donation, as predicted. Same f ...
Chapter 19 d-block metal chemistry: general considerations
... arrangements that give chirality; possess nonsuperimposable mirror images. 2) Diastereoisomers (geometric isomers): Isomers with differing spatial arrangements that result in different geometries (e.g. cis-trans). ...
... arrangements that give chirality; possess nonsuperimposable mirror images. 2) Diastereoisomers (geometric isomers): Isomers with differing spatial arrangements that result in different geometries (e.g. cis-trans). ...
Concepts in Transition Metal Chemistry – Questions
... complexes. What is the main bonding feature that molecular orbital theory uses to explain this for octahedral complexes? ...
... complexes. What is the main bonding feature that molecular orbital theory uses to explain this for octahedral complexes? ...
InorgCh14.1
... Ligand (CO) Substitution is important for synthesis of new complexes a) Rate is independent of incoming ligand = D mechanism (for most) Ni(CO)4 Ni(CO)3 18e- to 16e- (slow) Ni(CO)3 + L Ni(CO)3L 16e- to 18e- (fast) b) ...
... Ligand (CO) Substitution is important for synthesis of new complexes a) Rate is independent of incoming ligand = D mechanism (for most) Ni(CO)4 Ni(CO)3 18e- to 16e- (slow) Ni(CO)3 + L Ni(CO)3L 16e- to 18e- (fast) b) ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.