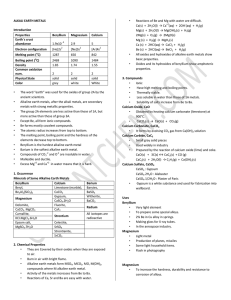
Document
... Nanoscience is the study of small particles that are between 1 and 100 nanometres in size 1 nanometre (1 nm) = 1 x 10-9 metres (0.000 000 001m or a billionth of a metre) Nanoparticles show different properties to the same materials in bulk and have a high surface area to volume ratio. This may lead ...
... Nanoscience is the study of small particles that are between 1 and 100 nanometres in size 1 nanometre (1 nm) = 1 x 10-9 metres (0.000 000 001m or a billionth of a metre) Nanoparticles show different properties to the same materials in bulk and have a high surface area to volume ratio. This may lead ...
Calixarene-Based Oxovanadium Complexes as Molecular Models
... Until recently, oxovanadium complexes of calix[4]arenes (C4–) were not known, and the literature on the coordination of oxovanadium units by other calixarene-based ligands was scarce, too: one complex with a ligand derived from H4C by monomethylation of a phenolic function, CMeVV=O (1), (see Scheme ...
... Until recently, oxovanadium complexes of calix[4]arenes (C4–) were not known, and the literature on the coordination of oxovanadium units by other calixarene-based ligands was scarce, too: one complex with a ligand derived from H4C by monomethylation of a phenolic function, CMeVV=O (1), (see Scheme ...
Article - Max Planck Institut für Festkörperforschung
... molecules do not point straight toward the center Fe. Rather, their triangular envelope is rotated by 22.5° clockwise or counterclockwise with respect to the principal axes of the compound. This is associated with a unidentate Fe-carboxylate bond, where one of the oxygen atoms of the carboxylate gro ...
... molecules do not point straight toward the center Fe. Rather, their triangular envelope is rotated by 22.5° clockwise or counterclockwise with respect to the principal axes of the compound. This is associated with a unidentate Fe-carboxylate bond, where one of the oxygen atoms of the carboxylate gro ...
this article (PDF 739.58 KB)
... geometric arrangement of metal atoms within limits does not unduly affect orbital overlap, thus allowing easy movement of metal atoms with respect to each other without breaking the lattice. The activation energy for such movement is small (-0.5 kJ/mol) compared to usual chemical processes (80 to 16 ...
... geometric arrangement of metal atoms within limits does not unduly affect orbital overlap, thus allowing easy movement of metal atoms with respect to each other without breaking the lattice. The activation energy for such movement is small (-0.5 kJ/mol) compared to usual chemical processes (80 to 16 ...
ppt Sc10 Review Notes
... to go from name to formula: first part is the same as before …look up the symbol for each ion then balance the charges using subscripts, then for the hydrate part…add “xH2O” where x is the number given in the prefix eg) iron (III) nitrate nonahydrate = Fe(NO3)39H2O ...
... to go from name to formula: first part is the same as before …look up the symbol for each ion then balance the charges using subscripts, then for the hydrate part…add “xH2O” where x is the number given in the prefix eg) iron (III) nitrate nonahydrate = Fe(NO3)39H2O ...
Ionic bonding - Nidderdale High School
... increases number of collisions and increases rate Temperature: Particles have more energy and move faster and collide more often. More particles have energy greater than the activation energy so more successful collisions Catalyst: Catalysts change the rate of chemical reactions but are not used up ...
... increases number of collisions and increases rate Temperature: Particles have more energy and move faster and collide more often. More particles have energy greater than the activation energy so more successful collisions Catalyst: Catalysts change the rate of chemical reactions but are not used up ...
instructor notes
... The difference between M(CO)6 and M(CO)5 is evident by IR and UV-Vis spectroscopy. The first set of images shows the IR and UV-Vis spectra of Cr(CO)6. After photolysis, Cr(CO)5 is produced as seen in the second set of spectra. Cr(CO)6 can be regenerated with further photolysis as seen in the final s ...
... The difference between M(CO)6 and M(CO)5 is evident by IR and UV-Vis spectroscopy. The first set of images shows the IR and UV-Vis spectra of Cr(CO)6. After photolysis, Cr(CO)5 is produced as seen in the second set of spectra. Cr(CO)6 can be regenerated with further photolysis as seen in the final s ...
transition-metals-colours-and-reactions
... molecule. One coordination site is left that can bind loosely to an oxygen molecule. Oxygen is a poor ligand that is easily released to cells, where its concentration is low. Ligands that can form stronger bonds with the Fe2+ ion, such as carbon monoxide, bind irreversibly and destroy haemoglobin’s ...
... molecule. One coordination site is left that can bind loosely to an oxygen molecule. Oxygen is a poor ligand that is easily released to cells, where its concentration is low. Ligands that can form stronger bonds with the Fe2+ ion, such as carbon monoxide, bind irreversibly and destroy haemoglobin’s ...
Chapters 6, 8
... If first atom is metal - ionic compound: 1. Find charges of both ions (from position in periodic table); write cation and anion with charges. 2. The sum of charges must be zero. Find out how many of each ion you must have. 3. Put index next to each ion indicating how many ions of that kind there is ...
... If first atom is metal - ionic compound: 1. Find charges of both ions (from position in periodic table); write cation and anion with charges. 2. The sum of charges must be zero. Find out how many of each ion you must have. 3. Put index next to each ion indicating how many ions of that kind there is ...
IOSR Journal of Applied Chemistry (IOSR-JAC)
... Synthesis of and Studies on 4-Methylpiperazine-1-carbamic Acid and Morpholinecarbamic... were incubated in water bath at 45oC for 10 minutes, removed from the water bath and cooled to room temperature. Eighty microliter (80µl) of p-nitrophenyl palmitate (p-NPP) substrate solution was added to each ...
... Synthesis of and Studies on 4-Methylpiperazine-1-carbamic Acid and Morpholinecarbamic... were incubated in water bath at 45oC for 10 minutes, removed from the water bath and cooled to room temperature. Eighty microliter (80µl) of p-nitrophenyl palmitate (p-NPP) substrate solution was added to each ...
Fall 2008 Blank Exam 1 - Department of Chemistry | Oregon State
... There are six significant figures in this measured quantity. There are five significant figures in this measured quantity. There are four significant figures in this measured quantity. There are three significant figures in this measured quantity. There are two significant figures in this measured q ...
... There are six significant figures in this measured quantity. There are five significant figures in this measured quantity. There are four significant figures in this measured quantity. There are three significant figures in this measured quantity. There are two significant figures in this measured q ...
Chapter 7
... • These are negatively charged ions resulting from a gain of electrons. • Nonmetals tend to add or share electrons into their highest occupied energy levels to become anions. This allows them to achieve an octet in their highest occupied energy level. • The charge for an anion is written with a numb ...
... • These are negatively charged ions resulting from a gain of electrons. • Nonmetals tend to add or share electrons into their highest occupied energy levels to become anions. This allows them to achieve an octet in their highest occupied energy level. • The charge for an anion is written with a numb ...
document
... Dissolving the chemicals in water helps them to react together faster. The water separates the chemicals into individual molecules or ions. The separate, free-floating particles come in contact more frequently so the reaction speeds up. ...
... Dissolving the chemicals in water helps them to react together faster. The water separates the chemicals into individual molecules or ions. The separate, free-floating particles come in contact more frequently so the reaction speeds up. ...
Get PDF - Wiley Online Library
... atoms. [b] The d10s0-type configuration is an excited state of the complex. [c] The d10s0 configuration is an excited state of the atomic metal fragment. Table 2). This trend originates directly from the s-donating capabilities of the ligands as reflected by the energy of the lone-pair orbital e(LP) ...
... atoms. [b] The d10s0-type configuration is an excited state of the complex. [c] The d10s0 configuration is an excited state of the atomic metal fragment. Table 2). This trend originates directly from the s-donating capabilities of the ligands as reflected by the energy of the lone-pair orbital e(LP) ...
cellular discrimination processes in metal accumulating cells
... Living systems have evolved in association with a wide variety of metal ions many of which are essential for the normal functioning of cells. It has, in fact, been argued that the fundamental involvement of iron- and copper-containing enzymes in respiratory pathways indicates that cells must have de ...
... Living systems have evolved in association with a wide variety of metal ions many of which are essential for the normal functioning of cells. It has, in fact, been argued that the fundamental involvement of iron- and copper-containing enzymes in respiratory pathways indicates that cells must have de ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.
















![[Mo 6 O 19 ] 2](http://s1.studyres.com/store/data/001711933_1-089685ece001fc7b794401458722b7ae-300x300.png)