AP Chapter 5
... • If the bodies are oppositely charged, one positive and one negative, they are attracted toward one another; if the bodies are similarly charged, both positive or both negative, the force between them is ...
... • If the bodies are oppositely charged, one positive and one negative, they are attracted toward one another; if the bodies are similarly charged, both positive or both negative, the force between them is ...
Chapter 7:The Quantum-Mechanical Model of
... Light is particle-like (E = hor E = hc/). Light has photons of quantized energy. Quantized energy can explain the emission of light from hot bodies, the emission of electrons from metal surfaces on which light shines (the photoelectric effect). Photoelectric Effect: Many metals emit electrons whe ...
... Light is particle-like (E = hor E = hc/). Light has photons of quantized energy. Quantized energy can explain the emission of light from hot bodies, the emission of electrons from metal surfaces on which light shines (the photoelectric effect). Photoelectric Effect: Many metals emit electrons whe ...
d - Solon City Schools
... While a beam of light has many wavelike characteristics, the beam can also be thought of as a stream of tiny particles or bundles of energy called photons. A photon is a particle of electromagnetic radiation with no rest mass that carries a quantum of energy. ...
... While a beam of light has many wavelike characteristics, the beam can also be thought of as a stream of tiny particles or bundles of energy called photons. A photon is a particle of electromagnetic radiation with no rest mass that carries a quantum of energy. ...
Electron Configuration
... As electron configurations can also be long and tedious, there is a shorthand form that conveys the same information ...
... As electron configurations can also be long and tedious, there is a shorthand form that conveys the same information ...
Honors Chemistry
... 39. The Lewis structures for the following molecules have all been drawn in previous questions. For each of these molecules, draw the structural formula to the proper shape and indicate if it is polar or nonpolar. If polar, draw the arrow indicating the direction of the dipole. ...
... 39. The Lewis structures for the following molecules have all been drawn in previous questions. For each of these molecules, draw the structural formula to the proper shape and indicate if it is polar or nonpolar. If polar, draw the arrow indicating the direction of the dipole. ...
1s 2s 2p - Solon City Schools
... can be any integer between 0 and n - 1. For n = 3, l can be either 0, 1, or 2. The magnetic quantum number (ml) can be any integer between -l and +l. For l = 2, m can be either -2, -1, 0, +1, +2. ...
... can be any integer between 0 and n - 1. For n = 3, l can be either 0, 1, or 2. The magnetic quantum number (ml) can be any integer between -l and +l. For l = 2, m can be either -2, -1, 0, +1, +2. ...
Quantum Theory and Atomic Structure
... – The energy profile of the emitted light could not be explained by the classical mechanics which assumes that the energy of an object can be continuously changed – Plank (1900) explained the energy profiles by assuming that the energy of an object can be changed only in discrete amounts (quanta) → ...
... – The energy profile of the emitted light could not be explained by the classical mechanics which assumes that the energy of an object can be continuously changed – Plank (1900) explained the energy profiles by assuming that the energy of an object can be changed only in discrete amounts (quanta) → ...
Text Book: Fundamentals of Physics Authors: Halliday, Resnick
... •Potential energies of the electron as a function of its position along the xaxis of the idealized trap. DIAGRAM An electron can be trapped in the U = 0. •When the electron is in the central cylinder, its potential energy U (=-eV) is zero because there the potential V is zero. •If the electron could ...
... •Potential energies of the electron as a function of its position along the xaxis of the idealized trap. DIAGRAM An electron can be trapped in the U = 0. •When the electron is in the central cylinder, its potential energy U (=-eV) is zero because there the potential V is zero. •If the electron could ...
Midterm Review
... Define the law of multiple proportions and provide examples of two compounds that illustrate the concept. ...
... Define the law of multiple proportions and provide examples of two compounds that illustrate the concept. ...
Atomic Structure Electrons in Atoms
... • The speed of a wave is a product of its wavelength and frequency: c = • Notice and are inversely proportional • In a vacuum, All electromagnetic waves travel at the speed of light (3.00 x 108 m/s) ...
... • The speed of a wave is a product of its wavelength and frequency: c = • Notice and are inversely proportional • In a vacuum, All electromagnetic waves travel at the speed of light (3.00 x 108 m/s) ...
Radiant Energy Electromagnetic Wave Electromagnetic Wave
... metal’s electrons • The greater the frequency, the more energy • If the photon has enough energy, the electron receives the energy and is ejected from the metal’s surface ...
... metal’s electrons • The greater the frequency, the more energy • If the photon has enough energy, the electron receives the energy and is ejected from the metal’s surface ...
Chapter 3 Electromagnetic Theory, Photons, and Light
... A laser pointer emits light at 630 nm in xy plane at =450 to axis x (counter clock-wise). The light is polarized along axis z , beam cross-section is A=1 mm2 and its power is P=1 mW. 1. Write an equation of E and B components of this EM wave for the region of the beam. z E ...
... A laser pointer emits light at 630 nm in xy plane at =450 to axis x (counter clock-wise). The light is polarized along axis z , beam cross-section is A=1 mm2 and its power is P=1 mW. 1. Write an equation of E and B components of this EM wave for the region of the beam. z E ...
Photon gas as a classical medium
... We have derived a relation between the diffusion time, tD and the time of condensation ...
... We have derived a relation between the diffusion time, tD and the time of condensation ...
Atomic structure review
... Thompson – discovered electrons Rutherford – discovered the nucleus – small dense positive nucleus, volume empty space Bohr – electrons have quantized (specific) energy, shell model Heisenberg – due to wave nature of electrons you can’t know the position and momentum of an electron at the same time ...
... Thompson – discovered electrons Rutherford – discovered the nucleus – small dense positive nucleus, volume empty space Bohr – electrons have quantized (specific) energy, shell model Heisenberg – due to wave nature of electrons you can’t know the position and momentum of an electron at the same time ...
Chemical Stoichiometry
... molecular formula of quinine. A Carbon atom is at each angle. Each C has 4 bonds (lines + Hs). Hs are not always drawn in & must be added. ...
... molecular formula of quinine. A Carbon atom is at each angle. Each C has 4 bonds (lines + Hs). Hs are not always drawn in & must be added. ...
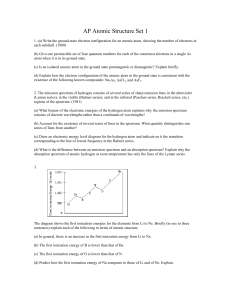
AP Atomic Structure Set 1
... 2. The emission spectrum of hydrogen consists of several series of sharp emission lines in the ultraviolet (Lyman series), in the visible (Balmer series), and in the infrared (Paschen series, Brackett series, etc,) regions of the spectrum. (1981) (a) What feature of the electronic energies of the hy ...
... 2. The emission spectrum of hydrogen consists of several series of sharp emission lines in the ultraviolet (Lyman series), in the visible (Balmer series), and in the infrared (Paschen series, Brackett series, etc,) regions of the spectrum. (1981) (a) What feature of the electronic energies of the hy ...
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical
... Let rot be the frequency of rotations (Cycles/second) The velocity of particle v=2πrrot= r ωrot where ωrot=2πrot has units of radians/second and is called the angular velocity. The kinetic energy of the revolving particle is: ...
... Let rot be the frequency of rotations (Cycles/second) The velocity of particle v=2πrrot= r ωrot where ωrot=2πrot has units of radians/second and is called the angular velocity. The kinetic energy of the revolving particle is: ...
Kvantfysik Lecture Notes No. 4x
... predictions for certain experiments using multi-electron atoms. In particular, it leads to beautiful predictions for the x-ray spectra of atoms with Z > 15. For now we make the following assumptions 1. The electrons are in discrete energy shells labeled by n = 1, 2, 3 . . .. 2. The lowest energy she ...
... predictions for certain experiments using multi-electron atoms. In particular, it leads to beautiful predictions for the x-ray spectra of atoms with Z > 15. For now we make the following assumptions 1. The electrons are in discrete energy shells labeled by n = 1, 2, 3 . . .. 2. The lowest energy she ...
Modern Physics
... If the chain reaction is controlled it can be used in a nuclear reactor If it is uncontrolled it explodes as a nuclear ...
... If the chain reaction is controlled it can be used in a nuclear reactor If it is uncontrolled it explodes as a nuclear ...
From electrons to quarks – the development of Particle Physics
... transition radiation (e.m. int.): when a charged particle crosses the boundary between two media with different speeds of light (different “refractive index”), e.m. radiation is emitted -- “transition radiation” amount of radiation grows with (energy/mass); bremsstrahlung (= braking radiation) ( ...
... transition radiation (e.m. int.): when a charged particle crosses the boundary between two media with different speeds of light (different “refractive index”), e.m. radiation is emitted -- “transition radiation” amount of radiation grows with (energy/mass); bremsstrahlung (= braking radiation) ( ...
Bremsstrahlung
Bremsstrahlung (German pronunciation: [ˈbʁɛmsˌʃtʁaːlʊŋ], from bremsen ""to brake"" and Strahlung ""radiation"", i.e. ""braking radiation"" or ""deceleration radiation"") is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into a photon, thus satisfying the law of conservation of energy. The term is also used to refer to the process of producing the radiation. Bremsstrahlung has a continuous spectrum, which becomes more intense and whose peak intensity shifts toward higher frequencies as the change of the energy of the accelerated particles increases.Strictly speaking, braking radiation is any radiation due to the acceleration of a charged particle, which includes synchrotron radiation, cyclotron radiation, and the emission of electrons and positrons during beta decay. However, the term is frequently used in the more narrow sense of radiation from electrons (from whatever source) slowing in matter.Bremsstrahlung emitted from plasma is sometimes referred to as free/free radiation. This refers to the fact that the radiation in this case is created by charged particles that are free both before and after the deflection (acceleration) that caused the emission.