Blackbody Radiation
... wave could not acquire any arbitrary amount of energy, but only allowed values that were multiples of a minimum wave energy. This quantum is given by h (or hc/), where h is constant = 6.63 x 10-34 J sec (Planck’s constant). Higher (shorter ) of wave, greater minimum energy. Short , high wave ...
... wave could not acquire any arbitrary amount of energy, but only allowed values that were multiples of a minimum wave energy. This quantum is given by h (or hc/), where h is constant = 6.63 x 10-34 J sec (Planck’s constant). Higher (shorter ) of wave, greater minimum energy. Short , high wave ...
The LPM effect in sequential bremsstrahlung
... gluon bremsstrahlung is the dominant process through which high energy particles lose energy when moving through the quark-gluon plasma. Naively, the bremsstrahlung rate will be roughly, ...
... gluon bremsstrahlung is the dominant process through which high energy particles lose energy when moving through the quark-gluon plasma. Naively, the bremsstrahlung rate will be roughly, ...
Chapter 4 Arrangements of Electrons in Atoms
... - electrons are in the ground state unless otherwise noted. -unfortunately, there is energy overlap beginning at n = 3. - How can we predict the sublevel order if this occurs? ...
... - electrons are in the ground state unless otherwise noted. -unfortunately, there is energy overlap beginning at n = 3. - How can we predict the sublevel order if this occurs? ...
Chapter 1 - Solutions
... Since the speed of light in a vacuum is independent of wavelength, it would take the same time for an infrared wave to travel the same distance. 6) (Burdge, 3.26) The blue color of the sky results from the scattering of sunlight from molecules of air. The blue light has a frequency of about 7.5 x 10 ...
... Since the speed of light in a vacuum is independent of wavelength, it would take the same time for an infrared wave to travel the same distance. 6) (Burdge, 3.26) The blue color of the sky results from the scattering of sunlight from molecules of air. The blue light has a frequency of about 7.5 x 10 ...
Section 1 - Tutor
... (a) Each atom has a dense central nucleus. (b) Electrons in atoms orbit the nucleus. (c) Each atom has a unique set of energy levels that electrons can move between. (d) Electrons in atoms are in constant motion. (e) Each atom is composed of positive and negative charges. ...
... (a) Each atom has a dense central nucleus. (b) Electrons in atoms orbit the nucleus. (c) Each atom has a unique set of energy levels that electrons can move between. (d) Electrons in atoms are in constant motion. (e) Each atom is composed of positive and negative charges. ...
Figure 4 - University of Wisconsin–Madison
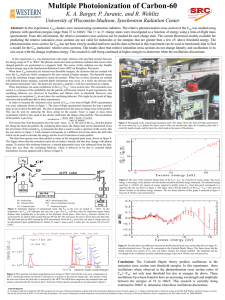
... photoionization cross section for C60 has not been closely studied until now and the data collected in this experiment can be used as benchmark data to find a model for the C60 molecules’ relative cross sections. The results show that relative ionization cross sections do not change linearly, and os ...
... photoionization cross section for C60 has not been closely studied until now and the data collected in this experiment can be used as benchmark data to find a model for the C60 molecules’ relative cross sections. The results show that relative ionization cross sections do not change linearly, and os ...
1 slide per page() - Wayne State University Physics and Astronomy
... includes some features of the currently accepted theory His model includes both classical and non-classical ideas His model included an attempt to explain why the atom was stable ...
... includes some features of the currently accepted theory His model includes both classical and non-classical ideas His model included an attempt to explain why the atom was stable ...
Chapter 7: Quantum Theory and the Electronic Structure of Atoms
... Erwin Schr6dinger developed a theory for computing electron energies based on the idea that we don’t have to specify the position or path of the electron in order to compute its energy and predict spectra. The Schr6dinger equation (SE) is beyond our ability to solve, but we will use the SE solutions ...
... Erwin Schr6dinger developed a theory for computing electron energies based on the idea that we don’t have to specify the position or path of the electron in order to compute its energy and predict spectra. The Schr6dinger equation (SE) is beyond our ability to solve, but we will use the SE solutions ...
P301_2009_Final_revi..
... key points since exam II. It also contains copies of the review slides you saw before exam I and II; it also •Time: Wed. 16 Dec. 2009 at 10:15 in the P301 classroom (SW 218) •Should be roughly 16 “questions” (6 points each, or ~60% longer than the mid-term exams). •Coverage: Comprehensive •Roughly 1 ...
... key points since exam II. It also contains copies of the review slides you saw before exam I and II; it also •Time: Wed. 16 Dec. 2009 at 10:15 in the P301 classroom (SW 218) •Should be roughly 16 “questions” (6 points each, or ~60% longer than the mid-term exams). •Coverage: Comprehensive •Roughly 1 ...
28 Quantum Physics
... A scanning electron microscope is used to look at cell structure with 10-‐nm resoluGon. A beam of electrons from the hot filament is accelerated with a voltage of 12 kV and then focused to a sm ...
... A scanning electron microscope is used to look at cell structure with 10-‐nm resoluGon. A beam of electrons from the hot filament is accelerated with a voltage of 12 kV and then focused to a sm ...
pdf file - HST
... For very high momenta, when p 2 c2 m20 c4 , E ≈ pc. (For photons, E = pc.) The relation p2 c2 m20 c4 holds for the particles discussed in this paper; they are ‘highly relativistic’ particles. For example: the positron approaching P has a momentum ∼ 200 MeV/c and a mass of 0.511 MeV/c2 ; so p2 c2 ...
... For very high momenta, when p 2 c2 m20 c4 , E ≈ pc. (For photons, E = pc.) The relation p2 c2 m20 c4 holds for the particles discussed in this paper; they are ‘highly relativistic’ particles. For example: the positron approaching P has a momentum ∼ 200 MeV/c and a mass of 0.511 MeV/c2 ; so p2 c2 ...
electron orbits atomic spectra the Bohr atom
... Bohr himself admitted his model didn’t explain anything. But it does tie together previously unexplained observations, and tells us the direction we might go in looking for the “true” model. See here for another triumph* of Bohr’s model: singly ionized helium (like hydrogen but with an extra neutro ...
... Bohr himself admitted his model didn’t explain anything. But it does tie together previously unexplained observations, and tells us the direction we might go in looking for the “true” model. See here for another triumph* of Bohr’s model: singly ionized helium (like hydrogen but with an extra neutro ...
Full Text Report
... HELIOS-CR solves the equation of motion for a single fluid. Electrons and ions are assumed to be co-moving. Pressure contributions to the equation of motion come from electrons, ions, radiation, and the magnetic field. Energy transport in the plasma can be treated using either a one-temperature ( Ti ...
... HELIOS-CR solves the equation of motion for a single fluid. Electrons and ions are assumed to be co-moving. Pressure contributions to the equation of motion come from electrons, ions, radiation, and the magnetic field. Energy transport in the plasma can be treated using either a one-temperature ( Ti ...
Document
... • Compare the wave and particle natures of light. • Define a quantum of energy, and explain how it is related to an energy change of matter. • Contrast continuous electromagnetic spectra and atomic emission spectra. ...
... • Compare the wave and particle natures of light. • Define a quantum of energy, and explain how it is related to an energy change of matter. • Contrast continuous electromagnetic spectra and atomic emission spectra. ...
C. - Taylor County Schools
... • Compare the wave and particle natures of light. • Define a quantum of energy, and explain how it is related to an energy change of matter. • Contrast continuous electromagnetic spectra and atomic emission spectra. radiation: the rays and particles —alpha particles, beta particles, and gamma rays—t ...
... • Compare the wave and particle natures of light. • Define a quantum of energy, and explain how it is related to an energy change of matter. • Contrast continuous electromagnetic spectra and atomic emission spectra. radiation: the rays and particles —alpha particles, beta particles, and gamma rays—t ...
Bremsstrahlung
Bremsstrahlung (German pronunciation: [ˈbʁɛmsˌʃtʁaːlʊŋ], from bremsen ""to brake"" and Strahlung ""radiation"", i.e. ""braking radiation"" or ""deceleration radiation"") is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into a photon, thus satisfying the law of conservation of energy. The term is also used to refer to the process of producing the radiation. Bremsstrahlung has a continuous spectrum, which becomes more intense and whose peak intensity shifts toward higher frequencies as the change of the energy of the accelerated particles increases.Strictly speaking, braking radiation is any radiation due to the acceleration of a charged particle, which includes synchrotron radiation, cyclotron radiation, and the emission of electrons and positrons during beta decay. However, the term is frequently used in the more narrow sense of radiation from electrons (from whatever source) slowing in matter.Bremsstrahlung emitted from plasma is sometimes referred to as free/free radiation. This refers to the fact that the radiation in this case is created by charged particles that are free both before and after the deflection (acceleration) that caused the emission.