Atomic Structure
... 1. Consider the human body as a system and apply the first law of thermodynamics to it. We know that over any given period of sufficient length (say one day), there will be a net heat flow from the body (i.e. Q is negative) and the body will do some external work on its surroundings (i.e. W is posit ...
... 1. Consider the human body as a system and apply the first law of thermodynamics to it. We know that over any given period of sufficient length (say one day), there will be a net heat flow from the body (i.e. Q is negative) and the body will do some external work on its surroundings (i.e. W is posit ...
Statistical - Jordan University of Science and Technology
... positions. Let ε be the energy necessary to remove an atom from a lattice site to an interstitial position and let n be the number of atoms occupying interstitial sites in equilibrium. a) what is the internal energy of the system. b) What is the total energy. Give an asymptotic formula when n>> 1? c ...
... positions. Let ε be the energy necessary to remove an atom from a lattice site to an interstitial position and let n be the number of atoms occupying interstitial sites in equilibrium. a) what is the internal energy of the system. b) What is the total energy. Give an asymptotic formula when n>> 1? c ...
Lecture 3 - McMaster Physics and Astronomy
... in any way that is convenient. Fourth, heat is not a property of a system like temperature, pressure, volume or mass. It is energy in transit – energy that enters or leaves a system as a consequence of a temperature difference between the system and a body with which it is in thermal contact (includ ...
... in any way that is convenient. Fourth, heat is not a property of a system like temperature, pressure, volume or mass. It is energy in transit – energy that enters or leaves a system as a consequence of a temperature difference between the system and a body with which it is in thermal contact (includ ...
Chapter Summary
... A cycle is a sequence of processes that returns a system to its original state. The cycle as a whole satisfies the first law of thermodynamics, as does each of its processes. The change in internal energy for any cycle is always zero, because the system returns to its initial state, and the area of ...
... A cycle is a sequence of processes that returns a system to its original state. The cycle as a whole satisfies the first law of thermodynamics, as does each of its processes. The change in internal energy for any cycle is always zero, because the system returns to its initial state, and the area of ...
Chapter 1: The first law of thermodynamics
... and volume we say that the quantity is a function of state. Therefore, for an ideal gas in equilibrium, the system’s temperature is a function of state ( θ = F ( P,V ) ). A quantity, dG, is said to be an exact differential if it only depends on the difference in the function of state between two clo ...
... and volume we say that the quantity is a function of state. Therefore, for an ideal gas in equilibrium, the system’s temperature is a function of state ( θ = F ( P,V ) ). A quantity, dG, is said to be an exact differential if it only depends on the difference in the function of state between two clo ...
PS5, Thermo Thermodynamics Standards: 3. Energy cannot be
... 1. Is the following sentence true or false? The flow of heat is not directly related to the flow of energy. 2. Circle the letter that best describes what happens when heat is added to a system. a. Much of it is destroyed immediately. b. It transforms to an equal amount of some other form of energy. ...
... 1. Is the following sentence true or false? The flow of heat is not directly related to the flow of energy. 2. Circle the letter that best describes what happens when heat is added to a system. a. Much of it is destroyed immediately. b. It transforms to an equal amount of some other form of energy. ...
Thermochemistry
... work done by the system work done on the system (on constant temperature) DE = q-PDV q = DE + PDV ...
... work done by the system work done on the system (on constant temperature) DE = q-PDV q = DE + PDV ...
Themodynamic notes section 6.1
... • The entropy of a system not in thermal equilibrium will increase. • The entropy of a system approaches a constant value as the system approaches absolute zero. ...
... • The entropy of a system not in thermal equilibrium will increase. • The entropy of a system approaches a constant value as the system approaches absolute zero. ...
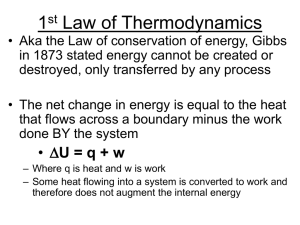
Internal energy is a characteristic of a given state – it is the same no
... you imagine the opposite happening: a glass of water spontaneously getting warm as an ice cube forms in its midst. That is perfectly consistent with the first law, but we never see it happen. The second law attempts to address this inconsistency. Several variations in statement: Heat will not sponta ...
... you imagine the opposite happening: a glass of water spontaneously getting warm as an ice cube forms in its midst. That is perfectly consistent with the first law, but we never see it happen. The second law attempts to address this inconsistency. Several variations in statement: Heat will not sponta ...
Heat

In physics, heat is energy in a process of transfer between a system and its surroundings, other than as work or with the transfer of matter. When there is a suitable physical pathway, heat flows from a hotter body to a colder one. The pathway can be direct, as in conduction and radiation, or indirect, as in convective circulation.Because it refers to a process of transfer between two systems, the system of interest, and its surroundings considered as a system, heat is not a state or property of a single system. If heat transfer is slow and continuous, so that the temperature of the system of interest remains well defined, it can sometimes be described by a process function.Kinetic theory explains heat as a macroscopic manifestation of the motions and interactions of microscopic constituents such as molecules and photons.In calorimetry, sensible heat is defined with respect to a specific chosen state variable of the system, such as pressure or volume. Sensible heat transferred into or out of the system under study causes change of temperature while leaving the chosen state variable unchanged. Heat transfer that occurs with the system at constant temperature and that does change that particular state variable is called latent heat with respect to that variable. For infinitesimal changes, the total incremental heat transfer is then the sum of the latent and sensible heat increments. This is a basic paradigm for thermodynamics, and was important in the historical development of the subject.The quantity of energy transferred as heat is a scalar expressed in an energy unit such as the joule (J) (SI), with a sign that is customarily positive when a transfer adds to the energy of a system. It can be measured by calorimetry, or determined by calculations based on other quantities, relying on the first law of thermodynamics.