chemistry i
... decreases. The equation E = hν means that as frequency increases, energy increases. Using this information and the reference tables, which color of visible light has the least energy? A. Red b. Yellow c. Green d. Violet 38. If an electron drops from n=6 to n=2, what type of electromagnetic radiation ...
... decreases. The equation E = hν means that as frequency increases, energy increases. Using this information and the reference tables, which color of visible light has the least energy? A. Red b. Yellow c. Green d. Violet 38. If an electron drops from n=6 to n=2, what type of electromagnetic radiation ...
PES Topography - Materials Computation Center
... Basis Sets (One-Particle) • Centered on atoms – this means we need fewer functions because geometry of molecule is embedded in basis set • Ideally, exponentially-decaying. This is the form of H atom solutions and is also the correct decay behavior for the density of a molecule. But then integrals a ...
... Basis Sets (One-Particle) • Centered on atoms – this means we need fewer functions because geometry of molecule is embedded in basis set • Ideally, exponentially-decaying. This is the form of H atom solutions and is also the correct decay behavior for the density of a molecule. But then integrals a ...
Chemistry Chapter 4 - Harding Charter Preparatory High School
... • The fixed energies an electron can have are called energy levels • To move from one energy level to another, an electron must gain or lose just the right amount of energy called a quantum – Thus, the energy of electron is said to be quantized – The energy levels are not equally spread, the higher ...
... • The fixed energies an electron can have are called energy levels • To move from one energy level to another, an electron must gain or lose just the right amount of energy called a quantum – Thus, the energy of electron is said to be quantized – The energy levels are not equally spread, the higher ...
Atomic Orbitals - Harding Charter Preparatory High School
... • The fixed energies an electron can have are called energy levels • To move from one energy level to another, an electron must gain or lose just the right amount of energy called a quantum – Thus, the energy of electron is said to be quantized – The energy levels are not equally spread, the higher ...
... • The fixed energies an electron can have are called energy levels • To move from one energy level to another, an electron must gain or lose just the right amount of energy called a quantum – Thus, the energy of electron is said to be quantized – The energy levels are not equally spread, the higher ...
Basis Sets - unix.eng.ua.edu
... 2. The HF wave functions may accurately describe other properties. Basis Sets: set of mathematical functions used to construct the wavefunction. • Each MO in HF theory is expressed as a linear combination of basis functions. • Full HF wavefunction is expressed as a Slater determinant formed from ind ...
... 2. The HF wave functions may accurately describe other properties. Basis Sets: set of mathematical functions used to construct the wavefunction. • Each MO in HF theory is expressed as a linear combination of basis functions. • Full HF wavefunction is expressed as a Slater determinant formed from ind ...
4 - College of Arts and Sciences
... Chap 2 - Atoms, Molecules and Ions {84Po210 Chap 3 - Stoichiometry {% yield, Limiting reagent etc Chap 4 - Solution Stoichiometry {Molarity; Solubility, Oxidation Chap 5 – Thermochemistry {Specific heat, Calorimetery, Hess’ Law Chap 6 - Electron configuration, Quantum numbers, Hund’s Rule ... ...
... Chap 2 - Atoms, Molecules and Ions {84Po210 Chap 3 - Stoichiometry {% yield, Limiting reagent etc Chap 4 - Solution Stoichiometry {Molarity; Solubility, Oxidation Chap 5 – Thermochemistry {Specific heat, Calorimetery, Hess’ Law Chap 6 - Electron configuration, Quantum numbers, Hund’s Rule ... ...
Chemistry Chapter 8 (HW Jan 28 Due Feb 5 Test Feb 6)
... ____ 27. In which of the following compounds is the octet expanded to include 12 electrons? a. H S c. PCl b. PCl d. SF ____ 28. How many electrons can occupy a single molecular orbital? a. 0 c. 2 b. 1 d. 4 ____ 29. How is a pair of molecular orbitals formed? a. by the splitting of a single atomic o ...
... ____ 27. In which of the following compounds is the octet expanded to include 12 electrons? a. H S c. PCl b. PCl d. SF ____ 28. How many electrons can occupy a single molecular orbital? a. 0 c. 2 b. 1 d. 4 ____ 29. How is a pair of molecular orbitals formed? a. by the splitting of a single atomic o ...
10.3 Ligand Field Theory 10.3 Ligand Field Theory
... - Fig.10.13. linear curve of the “corrected” enthalpies difference ...
... - Fig.10.13. linear curve of the “corrected” enthalpies difference ...
The study of biology can help you better understand
... How many energy sublevels are contained in each of the hydrogen atom’s first three energy levels? (5.2) ...
... How many energy sublevels are contained in each of the hydrogen atom’s first three energy levels? (5.2) ...
The role of atomic radius in ion channel selectivity :
... 3. Count the total # of e-s needed for each atom to have a full valence shell. 4. Subtract the number in step 2 (valence electrons) from the number in step 3 (total electrons for full shells). The result is the number of bonding electrons. 5. Assign 2 bonding electrons to each bond. 6. If bondin ...
... 3. Count the total # of e-s needed for each atom to have a full valence shell. 4. Subtract the number in step 2 (valence electrons) from the number in step 3 (total electrons for full shells). The result is the number of bonding electrons. 5. Assign 2 bonding electrons to each bond. 6. If bondin ...
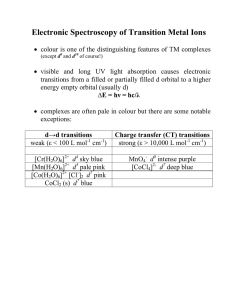
Electronic Spectroscopy of Transition Metal Ions
... orbitals so this gives rise to many possible states (RussellSaunders ‘terms’) that represent different energies for the system as a whole eg. d2 ion 1st electron: any of the 5 d-orbitals and spin up or down gives rise to 10 possibilities 2nd electron: can go in any d orbital but only spin paired in ...
... orbitals so this gives rise to many possible states (RussellSaunders ‘terms’) that represent different energies for the system as a whole eg. d2 ion 1st electron: any of the 5 d-orbitals and spin up or down gives rise to 10 possibilities 2nd electron: can go in any d orbital but only spin paired in ...
Chapter 5 Multiple Choice Questions
... as electrons jump from lower energy levels to higher levels. as the atoms condense from a gas to a liquid. as electrons jump from higher energy levels to lower levels. as they are heated and the solid melts to form a liquid. as the electrons move about the atom within an orbit. ...
... as electrons jump from lower energy levels to higher levels. as the atoms condense from a gas to a liquid. as electrons jump from higher energy levels to lower levels. as they are heated and the solid melts to form a liquid. as the electrons move about the atom within an orbit. ...
Notes on Electron Configurations
... Orbital Diagrams • p, d, and f orbitals are degenerate • Electrons will occupy separate orbitals, unpaired, before pairing up before pairing up • It takes more energy for an electron to occupy another subshell than it does to pair up The boxes are labeled with their subshell with their subsh ...
... Orbital Diagrams • p, d, and f orbitals are degenerate • Electrons will occupy separate orbitals, unpaired, before pairing up before pairing up • It takes more energy for an electron to occupy another subshell than it does to pair up The boxes are labeled with their subshell with their subsh ...
An element`s properties depend on the structure of its atoms
... Hybridization is the mixing of atomic orbitals in an atom to generate a set of new atomic orbitals called hybrid orbitals. • Mixing an s orbital with one of the p orbitals generates two equivalent sp hybrid orbitals. Note that the number of hybrid orbitals is equal to the number of atomic orbitals t ...
... Hybridization is the mixing of atomic orbitals in an atom to generate a set of new atomic orbitals called hybrid orbitals. • Mixing an s orbital with one of the p orbitals generates two equivalent sp hybrid orbitals. Note that the number of hybrid orbitals is equal to the number of atomic orbitals t ...
Molecular orbital

In chemistry, a molecular orbital (or MO) is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term orbital was introduced by Robert S. Mulliken in 1932 as an abbreviation for one-electron orbital wave function. At an elementary level, it is used to describe the region of space in which the function has a significant amplitude. Molecular orbitals are usually constructed by combining atomic orbitals or hybrid orbitals from each atom of the molecule, or other molecular orbitals from groups of atoms. They can be quantitatively calculated using the Hartree–Fock or self-consistent field (SCF) methods.