Atomic Theory electron charge: -1.6 X 10-19C
... researcher, Ernest Rutherford, provided clearer focus when he bombarded a thin sheet of gold foil with alpha rays (beams of helium nuclei). If atoms were uniformly dense, as he expected, all of the rays would have passed directly through. That did not occur. He recorded a few large deflections, very ...
... researcher, Ernest Rutherford, provided clearer focus when he bombarded a thin sheet of gold foil with alpha rays (beams of helium nuclei). If atoms were uniformly dense, as he expected, all of the rays would have passed directly through. That did not occur. He recorded a few large deflections, very ...
Chapter 8
... • The four regions of high electron density surrounding the oxygen tend to arrange themselves as far from each other as possible in order to minimize repulsive forces. This results in a tetrahedral geometry in which the H-O-H bond angle would be 109.5°. However, the two lone pairs around the oxygen ...
... • The four regions of high electron density surrounding the oxygen tend to arrange themselves as far from each other as possible in order to minimize repulsive forces. This results in a tetrahedral geometry in which the H-O-H bond angle would be 109.5°. However, the two lone pairs around the oxygen ...
Energy Level Models - Middle School Chemistry
... As the note on page 292 points out, there are other ways to model the electron energy levels of atoms. Some middle school texts show the electrons in pairs on an energy level. This pairing of electrons is intended to suggest information about the substructure within energy levels. This substructure ...
... As the note on page 292 points out, there are other ways to model the electron energy levels of atoms. Some middle school texts show the electrons in pairs on an energy level. This pairing of electrons is intended to suggest information about the substructure within energy levels. This substructure ...
Electron Structure of Atoms Notes
... EX: all orbitals that have n =3 are in the third shell. A collection of orbitals with the same n and ℓ values are in the same sub shell EX: 2s, 2p ...
... EX: all orbitals that have n =3 are in the third shell. A collection of orbitals with the same n and ℓ values are in the same sub shell EX: 2s, 2p ...
Zumdahl Chapter
... First Year Chemistry Podcast DVD Featuring Jonathan Bergmann and Aaron Sams from Peak Educational Consulting LLC All Rights Reserved © This is an interactive page that allows you to get to all of the content on this DVD. Click to each unit packet or podcast. The podcasts require Quicktime and the pa ...
... First Year Chemistry Podcast DVD Featuring Jonathan Bergmann and Aaron Sams from Peak Educational Consulting LLC All Rights Reserved © This is an interactive page that allows you to get to all of the content on this DVD. Click to each unit packet or podcast. The podcasts require Quicktime and the pa ...
Ch. 5 PPT Part 2
... 5.2 and 5.3: Bohr and the quantum mechanical model • Compare the Bohr and quantum mechanical models of the atom. • Explain the impact of de Broglie's wave article duality and the Heisenberg uncertainty principle on the current view of electrons in atoms. • Identify the relationships among a hydroge ...
... 5.2 and 5.3: Bohr and the quantum mechanical model • Compare the Bohr and quantum mechanical models of the atom. • Explain the impact of de Broglie's wave article duality and the Heisenberg uncertainty principle on the current view of electrons in atoms. • Identify the relationships among a hydroge ...
Introduction to Computational Chemistry
... In part II we have collected a number of subjects that we found interesting and that relate to somewhat more specialized techniques that belong to the toolbox of theoretically oriented chemist. We feel ...
... In part II we have collected a number of subjects that we found interesting and that relate to somewhat more specialized techniques that belong to the toolbox of theoretically oriented chemist. We feel ...
E. 2,3,6-trimethyl-4-octyne F. 1-butyl-3
... 6) (3 points) Hund’s rule states: a) Electrons fill orbitals starting at the lowest available (possible) energy states before filling higher states (e.g. 1s before 2s). b) No two identical fermions (particles with half-integer spin) may occupy the same quantum state simultaneously. c) Every orbital ...
... 6) (3 points) Hund’s rule states: a) Electrons fill orbitals starting at the lowest available (possible) energy states before filling higher states (e.g. 1s before 2s). b) No two identical fermions (particles with half-integer spin) may occupy the same quantum state simultaneously. c) Every orbital ...
Note 1.1 Chemistry of Life
... Orbital - is a region of space that is occupied by electrons located around the nucleus of an atom. The arrangement of electrons determines the chemical properties of an atom. Electrons are directly involved in the forming of breaking of bonds during chemical reactions. Electrons are found moving in ...
... Orbital - is a region of space that is occupied by electrons located around the nucleus of an atom. The arrangement of electrons determines the chemical properties of an atom. Electrons are directly involved in the forming of breaking of bonds during chemical reactions. Electrons are found moving in ...
Document
... • in experiments with the photoelectric effect, it was observed that there was a maximum wavelength for electrons to be emitted called the threshold frequency regardless of the intensity ...
... • in experiments with the photoelectric effect, it was observed that there was a maximum wavelength for electrons to be emitted called the threshold frequency regardless of the intensity ...
ON THE SHAPES OF ATOMS
... such as (effective) atomic radii. In the case of covalent molecular systems, conceptual models such as covalent radii, or physical models, for example the stick-and-ball models (or even the space-filling ones), also serve to cement that idea; when it is made clear that one cannot, in such circunstan ...
... such as (effective) atomic radii. In the case of covalent molecular systems, conceptual models such as covalent radii, or physical models, for example the stick-and-ball models (or even the space-filling ones), also serve to cement that idea; when it is made clear that one cannot, in such circunstan ...
Chapter 14: Phenomena Chapter 14 Covalent Bonding: Orbitals
... Phenomena: Scientists knew that in order to form a bond, orbitals on two atoms must overlap. However, px, py, and pz orbitals are located 90˚ from each other and compounds like CH4 (which would form bonds using their p orbitals) do not have bond angles of 90˚. Therefore, scientists had to explain th ...
... Phenomena: Scientists knew that in order to form a bond, orbitals on two atoms must overlap. However, px, py, and pz orbitals are located 90˚ from each other and compounds like CH4 (which would form bonds using their p orbitals) do not have bond angles of 90˚. Therefore, scientists had to explain th ...
Chem312 Au03 Problem Set 4
... because a photon can be absorbed by promotion of one electron from the t2g set of orbitals to the t2g eg set. In a diagram like the one at right, add ground state excited state electrons to represent the ground state and the lowest energy excited state. When you put the electrons in, you should foll ...
... because a photon can be absorbed by promotion of one electron from the t2g set of orbitals to the t2g eg set. In a diagram like the one at right, add ground state excited state electrons to represent the ground state and the lowest energy excited state. When you put the electrons in, you should foll ...
Exercises. 1.1 The power delivered to a photodetector which collects
... 3.8 A series of lines in the spectrum of atomic hydrogen lies at the wavelengths 656.46 nm, 486.27 nm, 434.17 nm, and 410.29 nm. What is the wavelength of the next line in the series? What energy is required to ionize the hydrogen atom when it is in the lower state involved in these transitions? 3.9 ...
... 3.8 A series of lines in the spectrum of atomic hydrogen lies at the wavelengths 656.46 nm, 486.27 nm, 434.17 nm, and 410.29 nm. What is the wavelength of the next line in the series? What energy is required to ionize the hydrogen atom when it is in the lower state involved in these transitions? 3.9 ...
Quantum Chemistry Predicts Multiply Bonded Diuranium
... The electronic configuration for the Cr-Cr bond in Cr2(OCHO)4 is (σg)2(πu)4(δg)2, a quadruple bond with a singlet ground state,21 but with a low-lying triplet state. The electronic configuration of U2 is (σg)2(πu)4 plus six electrons distributed among the remaining 6d and 5f orbitals. It is therefor ...
... The electronic configuration for the Cr-Cr bond in Cr2(OCHO)4 is (σg)2(πu)4(δg)2, a quadruple bond with a singlet ground state,21 but with a low-lying triplet state. The electronic configuration of U2 is (σg)2(πu)4 plus six electrons distributed among the remaining 6d and 5f orbitals. It is therefor ...
chemistry i - surrattchemistry
... 31. Which substance would have London dispersion forces as the main type of intermolecular forces of attraction? a. H2O b. F2 d. HCl d. NaCl 32. Diamond, graphite, and silicon dioxide all exhibit which type of intermolecular force? a. metallic b. network covalent c. ionic d. hydrogen e. dipole-dipol ...
... 31. Which substance would have London dispersion forces as the main type of intermolecular forces of attraction? a. H2O b. F2 d. HCl d. NaCl 32. Diamond, graphite, and silicon dioxide all exhibit which type of intermolecular force? a. metallic b. network covalent c. ionic d. hydrogen e. dipole-dipol ...
The Chemical Context of Life PPT
... If the electrons pile up on one portion of the three dimensional molecule, then we get a temporary concentration of negating charge, creating a temporary negative pole, if you will, since the electrons are not dispersed evenly we now refer to the molecule as a temporary dipole. ...
... If the electrons pile up on one portion of the three dimensional molecule, then we get a temporary concentration of negating charge, creating a temporary negative pole, if you will, since the electrons are not dispersed evenly we now refer to the molecule as a temporary dipole. ...
The Chemical Context of Life
... If the electrons pile up on one portion of the three dimensional molecule, then we get a temporary concentration of negating charge, creating a temporary negative pole, if you will, since the electrons are not dispersed evenly we now refer to the molecule as a temporary dipole. ...
... If the electrons pile up on one portion of the three dimensional molecule, then we get a temporary concentration of negating charge, creating a temporary negative pole, if you will, since the electrons are not dispersed evenly we now refer to the molecule as a temporary dipole. ...
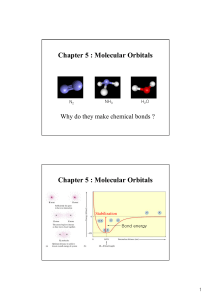
Molecular Orbitals Chapter 5 : Molecular Orbitals
... In contrast to LE, molecular orbitals describe how electrons spread over all the atoms in a molecule and bind them together, which can give correct views of • concept of resonance. • paramagnetic properties. • bond energy. Fact: O2 is paramagnetic! ...
... In contrast to LE, molecular orbitals describe how electrons spread over all the atoms in a molecule and bind them together, which can give correct views of • concept of resonance. • paramagnetic properties. • bond energy. Fact: O2 is paramagnetic! ...
Electrons!
... waves to describe the wave characteristics of material particles. This works only because the mass of an electron is so small. ...
... waves to describe the wave characteristics of material particles. This works only because the mass of an electron is so small. ...
Molecular orbital

In chemistry, a molecular orbital (or MO) is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term orbital was introduced by Robert S. Mulliken in 1932 as an abbreviation for one-electron orbital wave function. At an elementary level, it is used to describe the region of space in which the function has a significant amplitude. Molecular orbitals are usually constructed by combining atomic orbitals or hybrid orbitals from each atom of the molecule, or other molecular orbitals from groups of atoms. They can be quantitatively calculated using the Hartree–Fock or self-consistent field (SCF) methods.