0.08206 L atm/K mol - Arizona State University
... 59. A student calculated that 20.0 g of product should be obtained in a reaction. However, after doing the reaction, the student obtained only a 65.0% yield. What mass of product did the student actually recover? A. 13.0 g ...
... 59. A student calculated that 20.0 g of product should be obtained in a reaction. However, after doing the reaction, the student obtained only a 65.0% yield. What mass of product did the student actually recover? A. 13.0 g ...
13. Condensed azines. Quinoline. Isoquinoline. Acridine. Diazines
... colorless liquid with a boiling point of 208 °C. Pyridazine has no household use. It is mainly used in research and industry as building block for more complex compounds. The pyridazine structure is found within a number of herbicides such as credazine, pyridafol and pyridate. It is also found withi ...
... colorless liquid with a boiling point of 208 °C. Pyridazine has no household use. It is mainly used in research and industry as building block for more complex compounds. The pyridazine structure is found within a number of herbicides such as credazine, pyridafol and pyridate. It is also found withi ...
Experiment 22
... Although the product, [H+] [OH-] is small, that does not mean that both concentrations are necessarily small. If, for example, we dissolve HCl in water, the HCl in the solution will dissociate completely to H+ and Cl- ions; in 1 M HCl, [H+] will become 1 M, and there is nothing that Reaction 3 can ...
... Although the product, [H+] [OH-] is small, that does not mean that both concentrations are necessarily small. If, for example, we dissolve HCl in water, the HCl in the solution will dissociate completely to H+ and Cl- ions; in 1 M HCl, [H+] will become 1 M, and there is nothing that Reaction 3 can ...
Chemistry-Maths-Student-Guide
... but you’ve yet to put numbers into the idea. So why do chemists use maths? Mostly, because it’s good for describing patterns clearly and because it allows chemists to put a number on things: rather than saying something increases we’d like to be able to say by how much. This might mean that you find ...
... but you’ve yet to put numbers into the idea. So why do chemists use maths? Mostly, because it’s good for describing patterns clearly and because it allows chemists to put a number on things: rather than saying something increases we’d like to be able to say by how much. This might mean that you find ...
Kinetics and Mechanism of Uncatalyzed and Ag (I) Catalyzed
... N-bromo-benzenesulphonamide [11], in both acid and alkaline media have been studied. Although, various types of the reaction models have been suggested by different researchers [12-16], the specific details are yet to be discovered. Also, there are still controversies regarding the mechanistic pathw ...
... N-bromo-benzenesulphonamide [11], in both acid and alkaline media have been studied. Although, various types of the reaction models have been suggested by different researchers [12-16], the specific details are yet to be discovered. Also, there are still controversies regarding the mechanistic pathw ...
Structured questions
... ii) A component of the heavy fraction of petroleum has a molecular formula C12H26. In one of the reactions in Process 2, only a hydrocarbon and compound B are formed from this component. Write a chemical equation for the reaction involved. Catalytic hydration is employed to convert compound B into e ...
... ii) A component of the heavy fraction of petroleum has a molecular formula C12H26. In one of the reactions in Process 2, only a hydrocarbon and compound B are formed from this component. Write a chemical equation for the reaction involved. Catalytic hydration is employed to convert compound B into e ...
ch17
... K and Q for hetereogeneous equilibrium A hetereogeneous equilibrium involves reactants and/or products in different phases. A pure solid or liquid always has the same “concentration”, i.e., the same number of moles per liter of solid or liquid. The expressions for Q and K include only species whose ...
... K and Q for hetereogeneous equilibrium A hetereogeneous equilibrium involves reactants and/or products in different phases. A pure solid or liquid always has the same “concentration”, i.e., the same number of moles per liter of solid or liquid. The expressions for Q and K include only species whose ...
chemistry — released items - North Carolina Public Schools
... because the sample is transitioning from a gaseous state to a liquid state ...
... because the sample is transitioning from a gaseous state to a liquid state ...
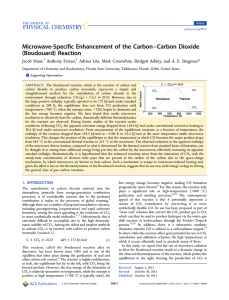
Microwave-Specific Enhancement of the Carbon−Carbon Dioxide
... comes up to temperature, the flow of CO2 commenced using the mass flow controller. As with microwave experiments, the volume of gas evolved during the reaction was measured using a totalizing mass flow meter. The product gas was sampled at the same 1 min intervals as in the microwave experiment and ana ...
... comes up to temperature, the flow of CO2 commenced using the mass flow controller. As with microwave experiments, the volume of gas evolved during the reaction was measured using a totalizing mass flow meter. The product gas was sampled at the same 1 min intervals as in the microwave experiment and ana ...
Unit-II - GDC Memorial College
... IUPAC nomenclature of branched and unbranched alkanes , the alkyl group, classi fication of carbon atoms in alkanes. Isomerism in alkanes, sources, methods of formation (with special reference to Wurtz reaction, Kolbe reaction, Corey-House reaction and decarboxylation of carboxylic acids), physical ...
... IUPAC nomenclature of branched and unbranched alkanes , the alkyl group, classi fication of carbon atoms in alkanes. Isomerism in alkanes, sources, methods of formation (with special reference to Wurtz reaction, Kolbe reaction, Corey-House reaction and decarboxylation of carboxylic acids), physical ...
Topic 4 - Lloyd Crosby
... A substance that does not ionize (does not produce any ions) when dissolved in water; a solution of a nonelectrolyte either does not conduct electricity at all or does so very poorly. 2. Most molecular substances are nonelectrolytes. MOLECULAR, COMPLETE IONIC, AND NET IONIC EQUATIONS A. Molecular eq ...
... A substance that does not ionize (does not produce any ions) when dissolved in water; a solution of a nonelectrolyte either does not conduct electricity at all or does so very poorly. 2. Most molecular substances are nonelectrolytes. MOLECULAR, COMPLETE IONIC, AND NET IONIC EQUATIONS A. Molecular eq ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.