Chapter 8
... – The formulas of the reactants and products must be correct. – The reactants are written to the left of the arrow and the products to the right of the arrow. ...
... – The formulas of the reactants and products must be correct. – The reactants are written to the left of the arrow and the products to the right of the arrow. ...
Chemistry I
... 28. For a gas with temperature and number of moles are held constant, Boyle’s law describes a situation in which: a. volume and pressure have no relationship b. volume increases with increasing pressure c. volume decreases with decreasing speed d. volume decreases with increasing pressure 29. The le ...
... 28. For a gas with temperature and number of moles are held constant, Boyle’s law describes a situation in which: a. volume and pressure have no relationship b. volume increases with increasing pressure c. volume decreases with decreasing speed d. volume decreases with increasing pressure 29. The le ...
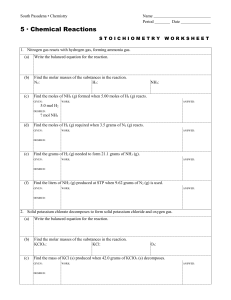
South Pasadena • Chemistry Name Period Date 5 · Chemical
... 4. A solution of lead acetate is combined with a solution of hydrochloric acid forming a lead chloride precipitate and acetic acid. (a) Write the balanced equation for the reaction. ...
... 4. A solution of lead acetate is combined with a solution of hydrochloric acid forming a lead chloride precipitate and acetic acid. (a) Write the balanced equation for the reaction. ...
Enzymology Lecture 5 - ASAB-NUST
... The plot of v versus [S] is not linear; although initially linear at low [S], it bends over to saturate at high [S]. Before the modern era of nonlinear curve-fitting on computers, this nonlinearity could make it difficult to estimate KM and Vmax accurately. Therefore, several researchers developed l ...
... The plot of v versus [S] is not linear; although initially linear at low [S], it bends over to saturate at high [S]. Before the modern era of nonlinear curve-fitting on computers, this nonlinearity could make it difficult to estimate KM and Vmax accurately. Therefore, several researchers developed l ...
Stoichiometry
... These reactions proceed if one of the ff. is satisfied: 1. An insoluble/slightly soluble product is formed (PRECIPITATE formation) 2. A weakly ionized species is produced. The most common species of this type is water. 3. A gas is produced as a product. ...
... These reactions proceed if one of the ff. is satisfied: 1. An insoluble/slightly soluble product is formed (PRECIPITATE formation) 2. A weakly ionized species is produced. The most common species of this type is water. 3. A gas is produced as a product. ...
Oxidation number and Electrolysis(電解)
... concentrated NaCl solution, only H + is discharged at the cathode. But if mercury electrode is used for the cathode, Na + is discharged because sodium metal forms an alloy with mercury. (This method is used in industry for the production of sodium.) ...
... concentrated NaCl solution, only H + is discharged at the cathode. But if mercury electrode is used for the cathode, Na + is discharged because sodium metal forms an alloy with mercury. (This method is used in industry for the production of sodium.) ...
Chapter 10
... charge on the ion of each element (metallic and nonmetallic) and form a compound from the two ions. If one of the elements forms more than one cation or 2 nonmetals are combined, the products can only be predicted if the ratio of elements in the compound are given ...
... charge on the ion of each element (metallic and nonmetallic) and form a compound from the two ions. If one of the elements forms more than one cation or 2 nonmetals are combined, the products can only be predicted if the ratio of elements in the compound are given ...
Types o.. - hrsbstaff.ednet.ns.ca
... from others). Gently heat with a Bunsen burner. (1) What do you observe on the upper part of the test tube? (2) What do you observe in the bottom of the test tube? Continue to heat until no further change is observed. Allow the test tube to cool, and add 5 drops of water. (3) What do you observe? (4 ...
... from others). Gently heat with a Bunsen burner. (1) What do you observe on the upper part of the test tube? (2) What do you observe in the bottom of the test tube? Continue to heat until no further change is observed. Allow the test tube to cool, and add 5 drops of water. (3) What do you observe? (4 ...
The origin and status of the Arrhenius equation
... the kinetics of reactions in solution an awareness of a more general shortcomine of the Arrhenius eouation in that experimental data, obiained with improved'precision and over wider ranaes of temperature. showed that in certain cases ~rrheniusblotswere -&deniably curved. Ironically, one of the first ...
... the kinetics of reactions in solution an awareness of a more general shortcomine of the Arrhenius eouation in that experimental data, obiained with improved'precision and over wider ranaes of temperature. showed that in certain cases ~rrheniusblotswere -&deniably curved. Ironically, one of the first ...
Chemical Equilibrium II
... Note that the equilibrium expression can be expressed by concentrations in terms of _________ for aqueous solutions or _________________ for gases (although for the purposes of Chemistry 12, we will not be using partial pressures) Some rules to follow when writing equilibrium expressions: “_________ ...
... Note that the equilibrium expression can be expressed by concentrations in terms of _________ for aqueous solutions or _________________ for gases (although for the purposes of Chemistry 12, we will not be using partial pressures) Some rules to follow when writing equilibrium expressions: “_________ ...
Chapter 3 – part I Sections 1-3
... • What is oxidized and reduced are always reactants, the products are the result of the redox. • So if asked “what is ox or red?”, answer is reactant ...
... • What is oxidized and reduced are always reactants, the products are the result of the redox. • So if asked “what is ox or red?”, answer is reactant ...
Class Syllabus
... geophysics, and mechanical engineering with an interest in characterizing and designing molecules, drugs, and materials. Courses in QM often focus more on applied mathematics rather than physical concepts. We start by understanding some of the essential differences between quantum and classical mech ...
... geophysics, and mechanical engineering with an interest in characterizing and designing molecules, drugs, and materials. Courses in QM often focus more on applied mathematics rather than physical concepts. We start by understanding some of the essential differences between quantum and classical mech ...
Fe(H2O)63+ + H2O → ← H3O+ + Fe(H2O)5(OH)2+
... 51. If a reaction proceeding by the mechanism A + B → C + D occurs at a rate x, and if the concentrations of A and B are both doubled, what will be the new rate of reaction? (A) (B) (C) (D) (E) ...
... 51. If a reaction proceeding by the mechanism A + B → C + D occurs at a rate x, and if the concentrations of A and B are both doubled, what will be the new rate of reaction? (A) (B) (C) (D) (E) ...
ch8 - Otterville R-VI School District
... Classify each of the following reactions one of the five basic types: Na2O + H2O NaOH Zn (s) + 2HCl(aq) ZnCl2(aq) + H2(g) Ca(s) + 2H2O(l) Ca(OH)2(aq) + H2(g) ...
... Classify each of the following reactions one of the five basic types: Na2O + H2O NaOH Zn (s) + 2HCl(aq) ZnCl2(aq) + H2(g) Ca(s) + 2H2O(l) Ca(OH)2(aq) + H2(g) ...
File
... as a unit. Energy is stored in chemical bonds. To break bonds, energy must be added. When bonds form, energy is released. All chemical reactions involve changes in energy. Energy is either produced or absorbed during a chemical reaction. For example, the burning of wood is a chemical reaction (see F ...
... as a unit. Energy is stored in chemical bonds. To break bonds, energy must be added. When bonds form, energy is released. All chemical reactions involve changes in energy. Energy is either produced or absorbed during a chemical reaction. For example, the burning of wood is a chemical reaction (see F ...
Chemistry 40S – Exam Review
... 2. Identify the conditions required for chemical equilibrium. 3. What statement is TRUE about a system at chemical equilibrium? a) observable changes occur during equilibrium b) the [ ]’s of reactants and products are equal c) the forward and reverse reaction rates are equal d) there are no reaction ...
... 2. Identify the conditions required for chemical equilibrium. 3. What statement is TRUE about a system at chemical equilibrium? a) observable changes occur during equilibrium b) the [ ]’s of reactants and products are equal c) the forward and reverse reaction rates are equal d) there are no reaction ...
Eötvös Loránd Science University Faculty of Sciences Department of
... Historical review of chemical kinetics. The scope of modern kinetics. Definition of the reaction rate and its formulation using different time derivatives. Collision theory in kinetics. Potential energy surfaces in reactive systems. The transition state theory based on quasi-equilibrium approach. Al ...
... Historical review of chemical kinetics. The scope of modern kinetics. Definition of the reaction rate and its formulation using different time derivatives. Collision theory in kinetics. Potential energy surfaces in reactive systems. The transition state theory based on quasi-equilibrium approach. Al ...
Exam 2 Review - Iowa State University
... Sodium Sulfide. Write the balanced equation. State the limiting reagent(s). How much precipitate forms? What is the molarity of the non-precipitate product? ...
... Sodium Sulfide. Write the balanced equation. State the limiting reagent(s). How much precipitate forms? What is the molarity of the non-precipitate product? ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.