2011-2012 ACAD REVIEW SHEET Chapter 16
... homogeneous equilibria heterogeneous equilibria reaction quotient Le Chatelier’s principle Haber process ...
... homogeneous equilibria heterogeneous equilibria reaction quotient Le Chatelier’s principle Haber process ...
GENERAL CHEMISTRY SECTION IV: THERMODYNAMICS
... system” can be hard because us humans are the surroundings, but we tend to think of things from our own perspectives. So if you’re thinking of whether you are losing or gaining something (like heat, for instance), you’ll be thinking of every sign in thermo backwards, because as the surroundings, if ...
... system” can be hard because us humans are the surroundings, but we tend to think of things from our own perspectives. So if you’re thinking of whether you are losing or gaining something (like heat, for instance), you’ll be thinking of every sign in thermo backwards, because as the surroundings, if ...
Topic/Objective - cloudfront.net
... Equilibrium constants for reactions in solution (1) Constants for acids and bases; pK; pH (2) Solubility product constants and their application to precipitation and the dissolution of slightly soluble compounds (3) Common ion effect; buffers; hydrolysis Unit 17 Organic Chemistry Introduction to o ...
... Equilibrium constants for reactions in solution (1) Constants for acids and bases; pK; pH (2) Solubility product constants and their application to precipitation and the dissolution of slightly soluble compounds (3) Common ion effect; buffers; hydrolysis Unit 17 Organic Chemistry Introduction to o ...
Energy and Chemical Reactions
... chloride and oxygen. 26. Explain why there is more energy released at constant pressure than at constant volume for the reaction that forms ammonia gas from nitrogen gas and hydrogen gas. 27. Explain why the heat at constant pressure is equal to the heat at constant volume for a reaction that forms ...
... chloride and oxygen. 26. Explain why there is more energy released at constant pressure than at constant volume for the reaction that forms ammonia gas from nitrogen gas and hydrogen gas. 27. Explain why the heat at constant pressure is equal to the heat at constant volume for a reaction that forms ...
Starter S-30
... Atoms – 2 atoms of nitrogen combine with 6 atoms of hydrogen – product is 2 nitrogen and 6 hydrogen Molecules – 1 molecule of nitrogen gas combines with 3 molecules of hydrogen gas to produce 2 molecules of ammonia ...
... Atoms – 2 atoms of nitrogen combine with 6 atoms of hydrogen – product is 2 nitrogen and 6 hydrogen Molecules – 1 molecule of nitrogen gas combines with 3 molecules of hydrogen gas to produce 2 molecules of ammonia ...
1999 Advanced Placement Chemistry Exam Section I: Multiple
... The energy change that occurs in the conversion 7. Represents an atom that has four valence elecof an ionic solid to widely separated gaseous trons ions 8. Represents an atom of a transition metal The energy in a chemical or physical change that is available to do useful work Questions 9–12 refer to ...
... The energy change that occurs in the conversion 7. Represents an atom that has four valence elecof an ionic solid to widely separated gaseous trons ions 8. Represents an atom of a transition metal The energy in a chemical or physical change that is available to do useful work Questions 9–12 refer to ...
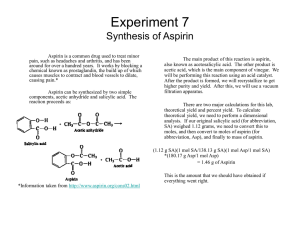
Experiment 7 Synthesis of Aspirin
... Aspirin is a common drug used to treat minor pain, such as headaches and arthritis, and has been around for over a hundred years. It works by blocking a chemical known as prostaglandin, the build up of which causes muscles to contract and blood vessels to dilate, causing pain.* Aspirin can be synthe ...
... Aspirin is a common drug used to treat minor pain, such as headaches and arthritis, and has been around for over a hundred years. It works by blocking a chemical known as prostaglandin, the build up of which causes muscles to contract and blood vessels to dilate, causing pain.* Aspirin can be synthe ...
Heats of Formation WS
... 7. The Ostwald process for the commercial production of nitric acid from ammonia and oxygen involves the following steps: 4 NH3 (g) + 5 O2 (g) 4 NO (g) + 6 H2O (g) 2 NO (g) + O2 (g) 2 NO2 (g) 3 NO2 (g) + H2O (l) 2 HNO3 (aq) + NO (g) [a] Use the values of ∆Hfº to calculate the value of ∆Hº for ...
... 7. The Ostwald process for the commercial production of nitric acid from ammonia and oxygen involves the following steps: 4 NH3 (g) + 5 O2 (g) 4 NO (g) + 6 H2O (g) 2 NO (g) + O2 (g) 2 NO2 (g) 3 NO2 (g) + H2O (l) 2 HNO3 (aq) + NO (g) [a] Use the values of ∆Hfº to calculate the value of ∆Hº for ...
Chapter 8 Chemical Equations and Reactions
... Write word and formula equations for the chemical reaction that occurs when solid sodium oxide is added to water at room temperature and forms sodium hydroxide (dissolved in the water). Include symbols for physical states in the formula equation. Then balance the formula equation to give a balanced ...
... Write word and formula equations for the chemical reaction that occurs when solid sodium oxide is added to water at room temperature and forms sodium hydroxide (dissolved in the water). Include symbols for physical states in the formula equation. Then balance the formula equation to give a balanced ...
Chemical Kinetics - mvhs
... time. Since the rates of reactions vary with time, this rate only gives an average over a period of time. It can be calculated by calculating change in concentration with time. Instantaneous Rate: Rate of reaction at ONE given point of time. It can be calculated from conc.- time graph by finding t ...
... time. Since the rates of reactions vary with time, this rate only gives an average over a period of time. It can be calculated by calculating change in concentration with time. Instantaneous Rate: Rate of reaction at ONE given point of time. It can be calculated from conc.- time graph by finding t ...
Major 1 Term 101 - KFUPM Faculty List
... and a mass of 12.1 grams. Will the block float in water and why? It will float, when its density is smaller than that of water (dwater = 1.00 g/cm3) density of the plastic block: dblock = m/V = 12.4 g/(2.2 x 3.0 x 1.5 cm3) = 1.3 g/cm3 Thus dblock > dwater and it will sink in water not float A) Yes, ...
... and a mass of 12.1 grams. Will the block float in water and why? It will float, when its density is smaller than that of water (dwater = 1.00 g/cm3) density of the plastic block: dblock = m/V = 12.4 g/(2.2 x 3.0 x 1.5 cm3) = 1.3 g/cm3 Thus dblock > dwater and it will sink in water not float A) Yes, ...
Lecture 1 and 2a - Thermochemistry
... Heat capacity is the heat required to raise an object by a number one degree Celsius. Larger objects have more heat capacity than smaller objects of the same composition. The units of heat capacity are J/°C or equivalently J/K. Specific heat capacity is the heat required to raise an object of one gr ...
... Heat capacity is the heat required to raise an object by a number one degree Celsius. Larger objects have more heat capacity than smaller objects of the same composition. The units of heat capacity are J/°C or equivalently J/K. Specific heat capacity is the heat required to raise an object of one gr ...
X273/13/02
... (c) The mass spectrum of compound A shows the molecular ion to have a mass/charge ratio of 74. Deduce the molecular formula of compound A. ...
... (c) The mass spectrum of compound A shows the molecular ion to have a mass/charge ratio of 74. Deduce the molecular formula of compound A. ...
Chemical Equations
... a) balance the different types of atoms ________________ b) first, balance the atoms of elements that are combined and that appear only ___________on each side of the equation c) balance ____________________________that appear on both sides of the equation as ...
... a) balance the different types of atoms ________________ b) first, balance the atoms of elements that are combined and that appear only ___________on each side of the equation c) balance ____________________________that appear on both sides of the equation as ...
Power Point Presentation
... until they collide with each other or the container walls: Explains how gas exerts a pressure - collisions with the container walls ...
... until they collide with each other or the container walls: Explains how gas exerts a pressure - collisions with the container walls ...
Formal balancing of chemical reaction networks
... Furthermore, since 1c L = 0 the sum of the rows of L is zero, and hence by the properties of the determinant function it directly follows that Cij does not depend on i; implying that Cij = ρj , j = 1, · · · , c. Therefore by defining ρ := (ρ1 , · · · , ρc )T , it follows from (4) that Lρ = 0. Furthe ...
... Furthermore, since 1c L = 0 the sum of the rows of L is zero, and hence by the properties of the determinant function it directly follows that Cij does not depend on i; implying that Cij = ρj , j = 1, · · · , c. Therefore by defining ρ := (ρ1 , · · · , ρc )T , it follows from (4) that Lρ = 0. Furthe ...
Tall: 1) The decomposition of CaCO3 is an endothermic process:
... A 1.00 mol sample of CO2 is heated to 1000K with excess graphite in a container of volume 40.0 L. At this temperature, Kc is 2.11x10-2 for the reaction: C(graphite) + CO2(g) 2 CO(g) a) b) ...
... A 1.00 mol sample of CO2 is heated to 1000K with excess graphite in a container of volume 40.0 L. At this temperature, Kc is 2.11x10-2 for the reaction: C(graphite) + CO2(g) 2 CO(g) a) b) ...
Answers to Homework Problem Sheet 8
... the enthalpy of formation of reactants and products: ΔrxnH° = ΣmΔfH°(products) - ΣnΔfH°(reactants) For reaction B this becomes: ΔrxnH° = [ΔfH°(B2O3(s) + 3ΔfH°(H2O(g))] – [ΔfH°(B2H6(g) + 3ΔfH°(O2(g))] Reaction A corresponds to the heat of formation of B2O3(s) so ΔfH°(B2O3(s)) = -1273 kJ mol-1. Reacti ...
... the enthalpy of formation of reactants and products: ΔrxnH° = ΣmΔfH°(products) - ΣnΔfH°(reactants) For reaction B this becomes: ΔrxnH° = [ΔfH°(B2O3(s) + 3ΔfH°(H2O(g))] – [ΔfH°(B2H6(g) + 3ΔfH°(O2(g))] Reaction A corresponds to the heat of formation of B2O3(s) so ΔfH°(B2O3(s)) = -1273 kJ mol-1. Reacti ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.