Biochemistry Assessment
... 1. Graph A. The increase in pressure increases the speed of the reaction. 2. Graph B. The increase in temperature increases the speed of the reaction. 3. Graph C. When temperature and pressure increase, the speed of the reaction increases more than with just temperature or pressure alone. H. Amino A ...
... 1. Graph A. The increase in pressure increases the speed of the reaction. 2. Graph B. The increase in temperature increases the speed of the reaction. 3. Graph C. When temperature and pressure increase, the speed of the reaction increases more than with just temperature or pressure alone. H. Amino A ...
chemical reaction
... • The word energy often is written in equations as either a reactant or a product. • Energy written as a reactant helps you think of energy as a necessary ingredient for the reaction to take place. Endothermic • Similarly, in the equation for an exothermic reaction, the word energy often is written ...
... • The word energy often is written in equations as either a reactant or a product. • Energy written as a reactant helps you think of energy as a necessary ingredient for the reaction to take place. Endothermic • Similarly, in the equation for an exothermic reaction, the word energy often is written ...
as a PDF
... amounts of o-donor ligands (e.g., pyridine, HMPA, 2,2-bipyridine, PPh3), or strong complexing olefins (e.g., 1,5 cyclooctadiene) were added at the beginning of the reaction of PPT with 1-hexene (1-hexene:PPT = 50,ligand:PPT = 2), the oxidation did not occur. The addition of pyridine, 2,2-bipyridine, ...
... amounts of o-donor ligands (e.g., pyridine, HMPA, 2,2-bipyridine, PPh3), or strong complexing olefins (e.g., 1,5 cyclooctadiene) were added at the beginning of the reaction of PPT with 1-hexene (1-hexene:PPT = 50,ligand:PPT = 2), the oxidation did not occur. The addition of pyridine, 2,2-bipyridine, ...
ppt
... Types of Chemical Reactions • Atoms and molecules react to create chemical reactions. • There are thousands of different chemical reactions, where atoms are never lost, just rearranged. ...
... Types of Chemical Reactions • Atoms and molecules react to create chemical reactions. • There are thousands of different chemical reactions, where atoms are never lost, just rearranged. ...
19-Oct
... How many kJ of energy are released when 23.7 g of hydrogen are reacted with excess chlorine to form hydrogen chloride. ...
... How many kJ of energy are released when 23.7 g of hydrogen are reacted with excess chlorine to form hydrogen chloride. ...
52.
... and sp2 centres. It is also necessary to extend experimental investigations of the rate-equilibrium behavior of alpha-nucleophilesto a range of dipolar non-hydroxylic solvents. Finally, it appears that the validity of the FM0 treatment of certain bimolecular reactions requires ...
... and sp2 centres. It is also necessary to extend experimental investigations of the rate-equilibrium behavior of alpha-nucleophilesto a range of dipolar non-hydroxylic solvents. Finally, it appears that the validity of the FM0 treatment of certain bimolecular reactions requires ...
Exam 2
... Calculate the energy, in kJ, released when 2.00 tonne (1 tonne = 106 gram) of coke is reacted with oxygen if 80% of the coke is oxidised to carbon dioxide and the remaining 20% is oxidised to carbon monoxide. ...
... Calculate the energy, in kJ, released when 2.00 tonne (1 tonne = 106 gram) of coke is reacted with oxygen if 80% of the coke is oxidised to carbon dioxide and the remaining 20% is oxidised to carbon monoxide. ...
Question paper - Edexcel
... the data booklet is not required. A SnO B SnO2 C SnBr2 D SnBr4 (Total for Question 8 = 1 mark) 9 What is the correct name for the molecule shown below? H3C ...
... the data booklet is not required. A SnO B SnO2 C SnBr2 D SnBr4 (Total for Question 8 = 1 mark) 9 What is the correct name for the molecule shown below? H3C ...
METO 637
... • Note that the rate of production of OH depends uniquely on the quantum yield of O1(D) as a function of wavelength. • Another source of atomic oxygen in the troposphere comes from the dissociation of nitrogen dioxide at wavelengths less than 400 nm: NO2 + hν → NO + O • The NO molecule can be oxidiz ...
... • Note that the rate of production of OH depends uniquely on the quantum yield of O1(D) as a function of wavelength. • Another source of atomic oxygen in the troposphere comes from the dissociation of nitrogen dioxide at wavelengths less than 400 nm: NO2 + hν → NO + O • The NO molecule can be oxidiz ...
Chirality in amino acid over layers at metal crystal surfaces
... particular enantiomer of the surface. For example, Cu{531}-S might offer a more suitable binding site for L-alanine than for D-alanine, and vice versa on Cu{531}-D. In fact, chiral fcc surfaces are highly prone to atomic-scale roughening, so this picture is too simplistic. However, the flexibility o ...
... particular enantiomer of the surface. For example, Cu{531}-S might offer a more suitable binding site for L-alanine than for D-alanine, and vice versa on Cu{531}-D. In fact, chiral fcc surfaces are highly prone to atomic-scale roughening, so this picture is too simplistic. However, the flexibility o ...
Chem 1711 Review Exam 2
... Enthalpy, ΔH: equate enthalpy change for a process to energy change for that process if it occurs at constant P; ΔH = qP ΔH = Hfinal — Hinitial ΔH associated with physical changes: ΔHvap, ΔHfus, ΔHsub where vap = vaporization, (g l), fus = fusion (l s), sub = sublimation (s g). This is not in ...
... Enthalpy, ΔH: equate enthalpy change for a process to energy change for that process if it occurs at constant P; ΔH = qP ΔH = Hfinal — Hinitial ΔH associated with physical changes: ΔHvap, ΔHfus, ΔHsub where vap = vaporization, (g l), fus = fusion (l s), sub = sublimation (s g). This is not in ...
Kinetics and Equilibrium Review Page 1
... B) greater than the rate of condensation C) equal to the rate of condensation D) equal to a zero rate of condensation 37. A liquid in a stoppered flask is allowed to stand at constant temperature until the liquid level in the flask remains constant. Which condition then exists in the flask? A) Only ...
... B) greater than the rate of condensation C) equal to the rate of condensation D) equal to a zero rate of condensation 37. A liquid in a stoppered flask is allowed to stand at constant temperature until the liquid level in the flask remains constant. Which condition then exists in the flask? A) Only ...
General, Organic, and Biological Chemistry
... 46) Isotopes are atoms of the same element that have A) different atomic numbers. B) the same atomic numbers but different numbers of protons. C) the same atomic numbers but different numbers of electrons. D) the same atomic number but different numbers of neutrons. E) the same atomic mass but diff ...
... 46) Isotopes are atoms of the same element that have A) different atomic numbers. B) the same atomic numbers but different numbers of protons. C) the same atomic numbers but different numbers of electrons. D) the same atomic number but different numbers of neutrons. E) the same atomic mass but diff ...
Organometallic Chemistry at the Magnesium− Tris (8
... ReceiVed December 6, 1999 Modern organic light emitting diode (OLED) devices are typically prepared in part by vapor phase deposition of a low work function metal cathode onto an organic electron carrier/ photoemitter. Tris(8-hydroxyquinolino)aluminum (Alq3, 1) is a commonly used electron carrier/ph ...
... ReceiVed December 6, 1999 Modern organic light emitting diode (OLED) devices are typically prepared in part by vapor phase deposition of a low work function metal cathode onto an organic electron carrier/ photoemitter. Tris(8-hydroxyquinolino)aluminum (Alq3, 1) is a commonly used electron carrier/ph ...
Practice Test 2
... acidity. In one analysis of a commercial vinegar brand, a 15.0 mL sample was titrated with 0.4500 M NaOH. It required 30.50 mL of this NaOH solution to neutralize the acid in the vinegar sample. What is the molar concentration of acetic acid in vinegar? A) B) C) D) ...
... acidity. In one analysis of a commercial vinegar brand, a 15.0 mL sample was titrated with 0.4500 M NaOH. It required 30.50 mL of this NaOH solution to neutralize the acid in the vinegar sample. What is the molar concentration of acetic acid in vinegar? A) B) C) D) ...
Lecture 2
... Hexaaminecobalt(III) chloride: [Co(NH3)6]Cl3 Number of ligand indicated by prefix (di,tri,tetra or bis, tris, tetrakis if ligand in parenthesis) tris(bipyridine)iron(II) chloride: [Fe(bipy)3]Cl2 Ligands named in alphabetical order ignoring prefix Anionic ligands are given the suffix -o (chloro-, sul ...
... Hexaaminecobalt(III) chloride: [Co(NH3)6]Cl3 Number of ligand indicated by prefix (di,tri,tetra or bis, tris, tetrakis if ligand in parenthesis) tris(bipyridine)iron(II) chloride: [Fe(bipy)3]Cl2 Ligands named in alphabetical order ignoring prefix Anionic ligands are given the suffix -o (chloro-, sul ...
Formal balancing of chemical reaction networks
... connected components of G are always strongly connected, or equivalently, that ρ as obtained from Kirchhoff’s Matrix Tree theorem is in Rc+ . Then define the undirected graph Ḡ as having the same vertices as G but half its number of edges, by replacing every pair of oppositely directed edges of G b ...
... connected components of G are always strongly connected, or equivalently, that ρ as obtained from Kirchhoff’s Matrix Tree theorem is in Rc+ . Then define the undirected graph Ḡ as having the same vertices as G but half its number of edges, by replacing every pair of oppositely directed edges of G b ...
Programma Inglese XXXII Scuola Corbella
... Getting the most from NMR - When chemical shift and couplings are just not enough ...
... Getting the most from NMR - When chemical shift and couplings are just not enough ...
South Pasadena • AP Chemistry
... 5. What three things must be taken into account when determining if a reaction has enough energy to overcome the activation energy (Ea)? 6. What does a catalyst do to a reaction? Explain. Something that is added to a reaction to speed up the reaction – it lowers the activation energy (energy necessa ...
... 5. What three things must be taken into account when determining if a reaction has enough energy to overcome the activation energy (Ea)? 6. What does a catalyst do to a reaction? Explain. Something that is added to a reaction to speed up the reaction – it lowers the activation energy (energy necessa ...
Thermodynamics and kinetics
... below ≈1E-5/mL no visible precipitate forms colloids • formation of supersaturated solutions slow kinetics • Competitive reactions may lower free ion concentration • Large excess of ligand may form soluble species AgCl(s) + Cl- <--> AgCl2-(aq) Ksp really best for slightly soluble salts ...
... below ≈1E-5/mL no visible precipitate forms colloids • formation of supersaturated solutions slow kinetics • Competitive reactions may lower free ion concentration • Large excess of ligand may form soluble species AgCl(s) + Cl- <--> AgCl2-(aq) Ksp really best for slightly soluble salts ...
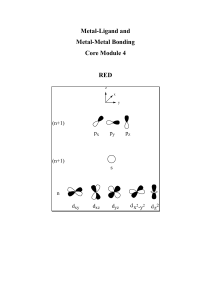
Metal-Ligand and Metal-Metal Bonding Lecture Notes
... use common nomenclature in transition metal chemistry. count valence electrons and determine metal oxidation state in transition metal complexes. Understand the physical basis of the 18-electron rule. appreciate the synergic nature of bonding in metal carbonyl complexes. understand the relationship ...
... use common nomenclature in transition metal chemistry. count valence electrons and determine metal oxidation state in transition metal complexes. Understand the physical basis of the 18-electron rule. appreciate the synergic nature of bonding in metal carbonyl complexes. understand the relationship ...
Hydrogen, Alkalis, and Alkaline Earths
... 2Na 0 2.2.2 cryptand Na cryptand Na ...
... 2Na 0 2.2.2 cryptand Na cryptand Na ...
Types of Chemical Reactions Name_________________________
... opportunity to learn about the different types of chemical reactions. The website address for this assignment is www.ric.edu/ptiskus/reactions. On the website you will find a brief description of the main types of chemical reactions. There are several representative reactions listed for each main ty ...
... opportunity to learn about the different types of chemical reactions. The website address for this assignment is www.ric.edu/ptiskus/reactions. On the website you will find a brief description of the main types of chemical reactions. There are several representative reactions listed for each main ty ...
Photoredox catalysis
_Schematic.png?width=300)
Photoredox catalysis is a branch of catalysis that harnesses the energy of visible light to accelerate a chemical reaction via a single-electron transfer. This area is named as a combination of ""photo-"" referring to light and redox, a condensed expression for the chemical processes of reduction and oxidation. In particular, photoredox catalysis employs small quantities of a light-sensitive compound that, when excited by light, can mediate the transfer of electrons between chemical compounds that otherwise would not react. Photoredox catalysts are generally drawn from three classes of materials: transition-metal complexes, organic dyes and semiconductors. While each class of materials has advantages, soluble transition-metal complexes are used most often.Study of this branch of catalysis led to the development of new methods to accomplish known and new chemical transformations. One attraction to the area is that photoredox catalysts are often less toxic than other reagents often used to generate free radicals, such as organotin reagents. Furthermore, while photoredox catalysts generate potent redox agents while exposed to light, they are innocuous under ordinary conditions Thus transition-metal complex photoredox catalysts are in some ways more attractive than stoichiometric redox agents such as quinones. The properties of photoredox catalysts can be modified by changing ligands and the metal, reflecting the somewhat modular nature of the catalyst.While photoredox catalysis has most often been applied to generate known reactive intermediates in a novel way, the study of this mode of catalysis led to the discovery of new organic reactions, such as the first direct functionalization of the β-arylation of saturated aldehydes. Although the D3-symmetric transition-metal complexes used in many photoredox-catalyzed reactions are chiral, the use of enantioenriched photoredox catalysts led to low levels of enantioselectivity in a photoredox-catalyzed aryl-aryl coupling reaction, suggesting that the chiral nature of these catalysts is not yet a highly effective means of transmitting stereochemical information in photoredox reactions. However, while synthetically useful levels of enantioselectivity have not been achieved using chiral photoredox catalysts alone, optically-active products have been obtained through the synergistic combination of photoredox catalysis with chiral organocatalysts such as secondary amines and Brønsted acids.