Chemistry for Changing Times
... Groups of atoms chemically bonded together H represents a hydrogen atom H2 represents a hydrogen molecule How many atoms of O are in H2O2? Be careful when writing formulas for ...
... Groups of atoms chemically bonded together H represents a hydrogen atom H2 represents a hydrogen molecule How many atoms of O are in H2O2? Be careful when writing formulas for ...
PHYSICAL SETTING CHEMISTRY
... Graphite and diamond are two crystalline arrangements for carbon. The crystal structure of graphite is organized in layers. The bonds between carbon atoms within each layer of graphite are strong. The bonds between carbon atoms that connect different layers of graphite are weak because the shared el ...
... Graphite and diamond are two crystalline arrangements for carbon. The crystal structure of graphite is organized in layers. The bonds between carbon atoms within each layer of graphite are strong. The bonds between carbon atoms that connect different layers of graphite are weak because the shared el ...
MSWord_examle - Magnetic Resonance in Solids
... Our work was initially stimulated by investigation of heavy-fermion Kondo lattice compounds. Very peculiar magnetic, thermal and transport properties of 4f-electron based heavy-fermion systems are determined by the interplay of the strong repulsion of 4f-electrons on the rare-earth ion sites, their ...
... Our work was initially stimulated by investigation of heavy-fermion Kondo lattice compounds. Very peculiar magnetic, thermal and transport properties of 4f-electron based heavy-fermion systems are determined by the interplay of the strong repulsion of 4f-electrons on the rare-earth ion sites, their ...
Chapter 2 - San Joaquin Memorial High School
... Although some chemists, including Dalton, objected to the new system, it was gradually adopted and forms the basis of the system we use today. In addition to these accomplishments, Berzelius discovered the elements cerium, thorium, selenium, and silicon. Of these elements, selenium and silicon are p ...
... Although some chemists, including Dalton, objected to the new system, it was gradually adopted and forms the basis of the system we use today. In addition to these accomplishments, Berzelius discovered the elements cerium, thorium, selenium, and silicon. Of these elements, selenium and silicon are p ...
CHEMISTRY-1 CHAPTER 8 CHEMICAL REACTIONS
... 1. Identify the type of reaction 2. Predict the product(s) using the type of reaction as a model 3. Balance it Don’t forget about the diatomic elements! (BrINClHOF) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a comp ...
... 1. Identify the type of reaction 2. Predict the product(s) using the type of reaction as a model 3. Balance it Don’t forget about the diatomic elements! (BrINClHOF) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a comp ...
Examples
... subscripts don’t effect the name if there is only one possibility still (cation)(anion) Aluminum oxide Potassium chloride ...
... subscripts don’t effect the name if there is only one possibility still (cation)(anion) Aluminum oxide Potassium chloride ...
Final Exam Review
... c. Definite volume; shape of container; no intermolecular attractions d. Volume and shape of container; no intermolecular attractions e. Volume and shape of container; strong intermolecular attractions 102. Which transformation is evaporation? a. liquid ---> solid d. solid ---> gas b. liquid ---> ga ...
... c. Definite volume; shape of container; no intermolecular attractions d. Volume and shape of container; no intermolecular attractions e. Volume and shape of container; strong intermolecular attractions 102. Which transformation is evaporation? a. liquid ---> solid d. solid ---> gas b. liquid ---> ga ...
AP Semester I Review: Free Response Questions
... water to produce 100. mL of solution. A 20.0 mL portion of the solution was titrated with KMnO4 (aq). The balanced equation for the reaction that occurred is as follows: 16 H+ (aq) + 2 MnO4- (aq) + 5 C2O42- (aq) 2 Mn2+ (aq) + 10 CO2 (g) + 8 H2O (l) The volume of 0.0150 M KMnO4 (aq) required to rea ...
... water to produce 100. mL of solution. A 20.0 mL portion of the solution was titrated with KMnO4 (aq). The balanced equation for the reaction that occurred is as follows: 16 H+ (aq) + 2 MnO4- (aq) + 5 C2O42- (aq) 2 Mn2+ (aq) + 10 CO2 (g) + 8 H2O (l) The volume of 0.0150 M KMnO4 (aq) required to rea ...
+2 - Fort Thomas Independent Schools
... 1. Write formulas for the binary ionic compounds formed between the following elements: a. potassium and iodine b. magnesium and chlorine c. sodium and sulfur d. aluminum and sulfur e. aluminum and nitrogen 2. Name the binary ionic compounds indicated by the following formulas: a. AgCl ...
... 1. Write formulas for the binary ionic compounds formed between the following elements: a. potassium and iodine b. magnesium and chlorine c. sodium and sulfur d. aluminum and sulfur e. aluminum and nitrogen 2. Name the binary ionic compounds indicated by the following formulas: a. AgCl ...
Chem1101 – Semester 1
... Orbitals of equal n nearest the nucleus have lowest energy: s < p < d < f... ...
... Orbitals of equal n nearest the nucleus have lowest energy: s < p < d < f... ...
F. The Quantum Atom Theory - River Dell Regional School District
... 5. Dalton’s Atomic Theory 1. Matter is made of small particles-atoms 2. Atoms of a given element are identical in size, mass, but differ from those of other elements*. 3. Atoms cannot be subdivided or destroyed*. ( supports law of conservation of mass) 4.Atoms combine in small whole number ratios t ...
... 5. Dalton’s Atomic Theory 1. Matter is made of small particles-atoms 2. Atoms of a given element are identical in size, mass, but differ from those of other elements*. 3. Atoms cannot be subdivided or destroyed*. ( supports law of conservation of mass) 4.Atoms combine in small whole number ratios t ...
Balanced Chemical Equation
... Follow the guidelines in this section of the text to assign oxidation numbers to all the elements in the following species: (a) H2S According to guideline 1, the oxidation number for H is +1. Using this oxidation number and the compound’s formula, guideline 4 may then be used to calculate the oxidat ...
... Follow the guidelines in this section of the text to assign oxidation numbers to all the elements in the following species: (a) H2S According to guideline 1, the oxidation number for H is +1. Using this oxidation number and the compound’s formula, guideline 4 may then be used to calculate the oxidat ...
Equation Chapter 1 Section 1 Tips for Studying: Take responsibility
... Naming Rules for an Ionic Compound: 1. Name the cation first by using the element’s name. 2. Name the anion second except minus the last syllable and replace it with “ide” I.e. NaCl = sodium chloride Writing Formulas for Ionic Compounds: ...
... Naming Rules for an Ionic Compound: 1. Name the cation first by using the element’s name. 2. Name the anion second except minus the last syllable and replace it with “ide” I.e. NaCl = sodium chloride Writing Formulas for Ionic Compounds: ...
CHE 0315 SEM 3, 2013/14 TOPIC 5: CHEMICAL BONDING 1. State
... By using the aid of appropriate model, describe the formation of metallic bond.[3]/5c The metal atoms contribute their valence electrons to form a sea of delocalized electron and the positive metal ions in an orderly array. A metallic bond is formed by the electrostatic attraction between the positi ...
... By using the aid of appropriate model, describe the formation of metallic bond.[3]/5c The metal atoms contribute their valence electrons to form a sea of delocalized electron and the positive metal ions in an orderly array. A metallic bond is formed by the electrostatic attraction between the positi ...
Chemical formula Chemistry Subscript Subscript
... 8U2 - 5(D) recognize that chemical formulas are used to identify substances and determine the number of atoms of each element in chemical formulas containing subscripts; A way of describing the number of atoms Chemical formula that makes up one molecule of a compound ...
... 8U2 - 5(D) recognize that chemical formulas are used to identify substances and determine the number of atoms of each element in chemical formulas containing subscripts; A way of describing the number of atoms Chemical formula that makes up one molecule of a compound ...
Document
... to those in the octahedral sites. The alignment of the spins is the result of an exchange interaction involving the O2− ions. The antiparallel alignment of the Fe3+ ion in the octahedral site and the Fe3+ ion in the tetrahedral site are usually explained by a superexchange reaction similar to that u ...
... to those in the octahedral sites. The alignment of the spins is the result of an exchange interaction involving the O2− ions. The antiparallel alignment of the Fe3+ ion in the octahedral site and the Fe3+ ion in the tetrahedral site are usually explained by a superexchange reaction similar to that u ...
Atoms Matter Energy Notes
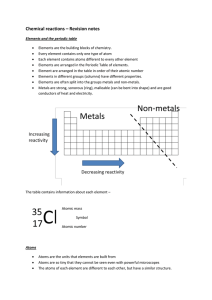
... o The periodic table is organized by physical properties. Element: a substance that cannot be broken down into smaller substances by ordinary chemical means. Atom: The basic particle from which all elements are made. Molecule: two or more atoms that are chemically combined to form a larger particle. ...
... o The periodic table is organized by physical properties. Element: a substance that cannot be broken down into smaller substances by ordinary chemical means. Atom: The basic particle from which all elements are made. Molecule: two or more atoms that are chemically combined to form a larger particle. ...
Chemistry 1. The Periodic Table displays the
... between electrons and protons and between atoms and molecules. As a basis for understanding this concept students know: a. atoms combine to form molecules by sharing electrons to form covalent or metallic bonds, or by exchanging electrons to form ionic bonds. b. chemical bonds between atoms in molec ...
... between electrons and protons and between atoms and molecules. As a basis for understanding this concept students know: a. atoms combine to form molecules by sharing electrons to form covalent or metallic bonds, or by exchanging electrons to form ionic bonds. b. chemical bonds between atoms in molec ...
For H 2 O
... we have by using the above prefixes. If we only have one of the first element listed, we do not need to state that by using the prefix mono-. However, we do need to state any other quantity of the elements. ...
... we have by using the above prefixes. If we only have one of the first element listed, we do not need to state that by using the prefix mono-. However, we do need to state any other quantity of the elements. ...
PHYSICAL SETTING CHEMISTRY
... At room temperature, a reaction occurs when KIO3(aq) is mixed with NaHSO3(aq) that contains a small amount of starch. The colorless reaction mixture turns dark blue after a period of time that depends on the concentration of the reactants. In a laboratory, 12 drops of a 0.02 M NaHSO3(aq) solution co ...
... At room temperature, a reaction occurs when KIO3(aq) is mixed with NaHSO3(aq) that contains a small amount of starch. The colorless reaction mixture turns dark blue after a period of time that depends on the concentration of the reactants. In a laboratory, 12 drops of a 0.02 M NaHSO3(aq) solution co ...
FE Exam Review for Chemistry
... What’s the difference between an atom and an isotope? Atoms have a defined standard number of neutrons. Number of neutrons = atomic mass – atomic number Isotopes have a non‐standard number of neutrons (heavy or light) How do you calculate average atomic mass? Average atomic mass is a weighted ave ...
... What’s the difference between an atom and an isotope? Atoms have a defined standard number of neutrons. Number of neutrons = atomic mass – atomic number Isotopes have a non‐standard number of neutrons (heavy or light) How do you calculate average atomic mass? Average atomic mass is a weighted ave ...
General, Organic, and Biological Chemistry
... Identify the letter of the diagram corresponding to the given type of reaction. ...
... Identify the letter of the diagram corresponding to the given type of reaction. ...
chapter 23 the transition elements and their
... Name nickel as nickel(II) to indicate oxidation state. Ligands are six (hexa-) waters (aqua). Put together with chloride anions to give hexaaquanickel(II) chloride. b) The cation is [Cr(en)3]n+ and the anion is ClO4–, the perchlorate ion. The charge on the cation is +3 to make a neutral salt in comb ...
... Name nickel as nickel(II) to indicate oxidation state. Ligands are six (hexa-) waters (aqua). Put together with chloride anions to give hexaaquanickel(II) chloride. b) The cation is [Cr(en)3]n+ and the anion is ClO4–, the perchlorate ion. The charge on the cation is +3 to make a neutral salt in comb ...
Slide 1 - Effingham County Schools
... • Aristotle was wrong. However, his theory persisted for 2000 years. ...
... • Aristotle was wrong. However, his theory persisted for 2000 years. ...