Ch 7: Reactions
... • Scientists have recognized several tendencies in reactants the DRIVE them to form products. ...
... • Scientists have recognized several tendencies in reactants the DRIVE them to form products. ...
Chem Final Study Guide Energy How much heat energy must be
... a) Alkali Metals (Group 1A) +1, Alkaline Metals (Group 2A) +2, Halogens (Group 7A) -1, Noble gases (Group 8A) No charges, Transition Metals (Group Bs) variable charges, Inner Transition Metals (Rows at bottom of periodic table) variable charges. 48) List at least 2 similarities for each family. a) O ...
... a) Alkali Metals (Group 1A) +1, Alkaline Metals (Group 2A) +2, Halogens (Group 7A) -1, Noble gases (Group 8A) No charges, Transition Metals (Group Bs) variable charges, Inner Transition Metals (Rows at bottom of periodic table) variable charges. 48) List at least 2 similarities for each family. a) O ...
Viscosity activation energy
... 1473 K in dependence of the molar fraction x of the modifying oxides. Most of the approaches are based on sophisticated models determining the average activation energy. Afterwards, the viscosity is
determined by introducing the value of into
Equation (4). The result is illustrated by a dott ...
... 1473 K in dependence of the molar fraction x of the modifying oxides. Most of the approaches are based on sophisticated models determining the average activation energy
2 - CronScience
... Rules for balancing: 1) Assemble the correct formulas for all the reactants and products, using “+” and “→” 2) Count the number of atoms of each type appearing on both sides 3) Balance the elements one at a time by adding coefficients (the numbers in front) where you need more - save balancing the ...
... Rules for balancing: 1) Assemble the correct formulas for all the reactants and products, using “+” and “→” 2) Count the number of atoms of each type appearing on both sides 3) Balance the elements one at a time by adding coefficients (the numbers in front) where you need more - save balancing the ...
1. What are micelles? Give two examples of micellar systems. Sol. A
... energetically preferred orientation has the magnetic moment aligned parallel with the applied field (spin +1/2) and is often given the notation , whereas the higher energy anti-parallel orientation (spin -1/2) is referred to as . The rotational axis of the spinning nucleus cannot be orientated exact ...
... energetically preferred orientation has the magnetic moment aligned parallel with the applied field (spin +1/2) and is often given the notation , whereas the higher energy anti-parallel orientation (spin -1/2) is referred to as . The rotational axis of the spinning nucleus cannot be orientated exact ...
Tall: 1) The decomposition of CaCO3 is an endothermic process:
... The reaction below has an equilibrium constant, Keq, of 171 at 25oC. Using the reaction conditions given, determine if the reaction is product-favored, reactant-favored, or at equilibrium. Don’t forget to find Molarity first! 2 NO2(g) N2O4(g) a) 2.0x10-3 mol NO2, 1.5x10-5 mol N2O4, 10.0 L flask ...
... The reaction below has an equilibrium constant, Keq, of 171 at 25oC. Using the reaction conditions given, determine if the reaction is product-favored, reactant-favored, or at equilibrium. Don’t forget to find Molarity first! 2 NO2(g) N2O4(g) a) 2.0x10-3 mol NO2, 1.5x10-5 mol N2O4, 10.0 L flask ...
Lecture 5
... • Determine the type of reaction and formulae of the products • Write an unbalanced equation with the correct reactants and products • Balance the equation by the use of prefixes (coefficients) to balance the number of each type of atom on the reactant and product sides of the equation. ...
... • Determine the type of reaction and formulae of the products • Write an unbalanced equation with the correct reactants and products • Balance the equation by the use of prefixes (coefficients) to balance the number of each type of atom on the reactant and product sides of the equation. ...
Final Exam review semester 1
... The reaction in Figure 7-1 shows the formation of ammonia from nitrogen and hydrogen in the Haber process. What will be the effect on the equilibrium if the temperature is increased and some of the ammonia is removed from the system? ____ ____ ...
... The reaction in Figure 7-1 shows the formation of ammonia from nitrogen and hydrogen in the Haber process. What will be the effect on the equilibrium if the temperature is increased and some of the ammonia is removed from the system? ____ ____ ...
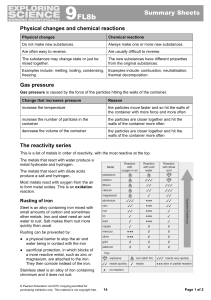
9F Reactivity - Parrs Wood High School
... Steel is an alloy containing iron mixed with small amounts of carbon and sometimes other metals. Iron and steel need air and water to rust. Salt makes them rust more quickly than usual. Rusting can be prevented by: ● a physical barrier to stop the air and water being in contact with the iron ● sacri ...
... Steel is an alloy containing iron mixed with small amounts of carbon and sometimes other metals. Iron and steel need air and water to rust. Salt makes them rust more quickly than usual. Rusting can be prevented by: ● a physical barrier to stop the air and water being in contact with the iron ● sacri ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.