Experiment 1 - Melting Points - NAU jan.ucc.nau.edu web server
... The melting point of a substance (the temperature at which a substance melts) is a physical property that can be used for its identification. It is a measure of the amount of kinetic energy (heat) that must be supplied to the particles of the substance in order to overcome the intermolecular forces ...
... The melting point of a substance (the temperature at which a substance melts) is a physical property that can be used for its identification. It is a measure of the amount of kinetic energy (heat) that must be supplied to the particles of the substance in order to overcome the intermolecular forces ...
CHOICE BASED CREDIT SYSTEM B. Sc. WITH CHEMISTRY
... Atomic Structure: Review of: Bohr’s theory and its limitations, dual behaviour of matter and radiation, de Broglie’s relation, Heisenberg Uncertainty principle. Hydrogen atom spectra. Need of a new approach to Atomic structure. What is Quantum mechanics? Time independent Schrodinger equation and mea ...
... Atomic Structure: Review of: Bohr’s theory and its limitations, dual behaviour of matter and radiation, de Broglie’s relation, Heisenberg Uncertainty principle. Hydrogen atom spectra. Need of a new approach to Atomic structure. What is Quantum mechanics? Time independent Schrodinger equation and mea ...
Chemical Vapor Deposition of Si and SiGe Films for High
... integration. Both processes are based on chemical vapor deposition (CVD) of Si. The emitter is prepared using in situ phosphorus (P) doping of poly-Si and the base is fabricated using non-selective epitaxial growth (NSEG) of Si and SiGe. The poly-Si emitter and epitaxial SiGe base studied in this th ...
... integration. Both processes are based on chemical vapor deposition (CVD) of Si. The emitter is prepared using in situ phosphorus (P) doping of poly-Si and the base is fabricated using non-selective epitaxial growth (NSEG) of Si and SiGe. The poly-Si emitter and epitaxial SiGe base studied in this th ...
4) What is the term for the procedure of collecting data and recording
... What is the term for a change that requires altering the composition of a substance? A) atomic change B) chemical change C) molecular change D) physical change E) none of the above What is the term for the symbolic representation of a compound that indicates the number of atoms of each element in th ...
... What is the term for a change that requires altering the composition of a substance? A) atomic change B) chemical change C) molecular change D) physical change E) none of the above What is the term for the symbolic representation of a compound that indicates the number of atoms of each element in th ...
PDF file - Comp Chem - University of Minnesota Twin Cities
... its structure, and nanoparticles are no exception to this generalization. We therefore rst ask, for nanoparticles of given size N, which structure is the one that is most likely to be prepared and observed in experiments. To answer this question is not easy for two reasons. First, the observed stru ...
... its structure, and nanoparticles are no exception to this generalization. We therefore rst ask, for nanoparticles of given size N, which structure is the one that is most likely to be prepared and observed in experiments. To answer this question is not easy for two reasons. First, the observed stru ...
Table of Contents
... gets more and more certain. The hypothesis becomes a _______________________, which is a thoroughly tested model that explains why things behave a certain way. Theories can never be ____________________; they are always subject to additional research. Another outcome is that certain behavior is repe ...
... gets more and more certain. The hypothesis becomes a _______________________, which is a thoroughly tested model that explains why things behave a certain way. Theories can never be ____________________; they are always subject to additional research. Another outcome is that certain behavior is repe ...
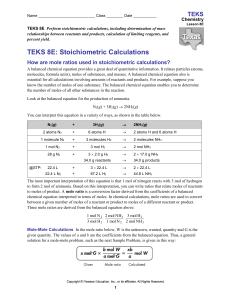
TEKS 5 - Online Learning Exchange
... Before the reaction takes place, nitrogen and hydrogen are present in a 2:3 molecule (mole) ratio. The reaction takes place according to the balanced equation. One molecule (mole) of N2 reacts with three molecules (moles) of H2 to produce two molecules (moles) of NH3. At this point, all the hydrogen ...
... Before the reaction takes place, nitrogen and hydrogen are present in a 2:3 molecule (mole) ratio. The reaction takes place according to the balanced equation. One molecule (mole) of N2 reacts with three molecules (moles) of H2 to produce two molecules (moles) of NH3. At this point, all the hydrogen ...
orange review book_2014_key
... 19. One similarity between all mixtures and compounds is that both (1) are heterogeneous (2) consist of two or more substances (3) are homogeneous (4) are heterogeneous 20. A dilute, aqueous potassium nitrate solution is best classified as a (1) homogeneous compound (2) homogeneous mixture ...
... 19. One similarity between all mixtures and compounds is that both (1) are heterogeneous (2) consist of two or more substances (3) are homogeneous (4) are heterogeneous 20. A dilute, aqueous potassium nitrate solution is best classified as a (1) homogeneous compound (2) homogeneous mixture ...
Reliable Computation of Equilibrium States and Bifurcations in Food
... solutions, and so the total number of equilibrium states may be unknown a priori. For simple models, it may be possible to solve for many of the equilibrium states analytically, but for more complex models a computational method is needed that is capable of finding, with certainty, all the solutions ...
... solutions, and so the total number of equilibrium states may be unknown a priori. For simple models, it may be possible to solve for many of the equilibrium states analytically, but for more complex models a computational method is needed that is capable of finding, with certainty, all the solutions ...
Kinetic Assay of Human Pepsin with Albumin
... by studying the time course of the pepsinsubstrate reaction. Km and V, were calculated by using the Hanes plot (7). We also examined the effect of temperature on the reaction and constructed an Arrhenius plot of the data relating temperature to pepsin activity, from which we calculated the activatio ...
... by studying the time course of the pepsinsubstrate reaction. Km and V, were calculated by using the Hanes plot (7). We also examined the effect of temperature on the reaction and constructed an Arrhenius plot of the data relating temperature to pepsin activity, from which we calculated the activatio ...
General and Inorganic Chemistry
... 4. IV Gas Laws ................................................................................................................................. 57 1. IV.1 The gas state ................................................................................................................ 57 1.1. IV.1.1 Th ...
... 4. IV Gas Laws ................................................................................................................................. 57 1. IV.1 The gas state ................................................................................................................ 57 1.1. IV.1.1 Th ...
containing complexes of aromatic amino acids
... All calculations were performed using the Gaussian03 quantum chemical program.17 The total energies of Cu(II) complexes and radical cations were calculated by the unrestricted open-shell formalism within the framework of Becke’s three-parameter DFT hybrid functional, B3LYP, which is based on a mixtu ...
... All calculations were performed using the Gaussian03 quantum chemical program.17 The total energies of Cu(II) complexes and radical cations were calculated by the unrestricted open-shell formalism within the framework of Becke’s three-parameter DFT hybrid functional, B3LYP, which is based on a mixtu ...
Preparatory Problems of the 40th IChO - IChO-2016
... ’Not at all, Watson. Have you ever seen a poison in so big a pellet? It would hardly be healthy to swallow, but that is not the point. Now look at this.’ He took out a pellet, dried it with great care, and dropped it into a bowl of water. Instead of slowly dissolving or sinking, the pellet began a s ...
... ’Not at all, Watson. Have you ever seen a poison in so big a pellet? It would hardly be healthy to swallow, but that is not the point. Now look at this.’ He took out a pellet, dried it with great care, and dropped it into a bowl of water. Instead of slowly dissolving or sinking, the pellet began a s ...
5. Coenzyme HAD+ is derived
... systems. Therefore, medical students must thoroughly understand the basic ideas, laws and methods of this science. Program expected to consider the foundations of the most important topics of the course of inorganic, organic, physical, colloid chemistry. Discipline "Chemistry" is preparing a theoret ...
... systems. Therefore, medical students must thoroughly understand the basic ideas, laws and methods of this science. Program expected to consider the foundations of the most important topics of the course of inorganic, organic, physical, colloid chemistry. Discipline "Chemistry" is preparing a theoret ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.