Review Packet - Daigneault Chem.is.try
... Organize and review all old exams and reading journals. Study sequentially. Divide your study time into short, intense sections. This can be more effective than studying continually for a long period of time. “Guess the test questions”. You should ask yourself what is most important when stu ...
... Organize and review all old exams and reading journals. Study sequentially. Divide your study time into short, intense sections. This can be more effective than studying continually for a long period of time. “Guess the test questions”. You should ask yourself what is most important when stu ...
Chem 173: Final Exam Review Short Answer and Problems 1
... Limestone is composed of calcium carbonate (CaCO3) as well as other compounds. In an analysis, a chemist takes a sample of limestone which has a mass of 413 mg and treats it with oxalic acid (H2C 2O4). A chemical reaction occurs between the calcium carbonate and the acid producing calcium oxalate an ...
... Limestone is composed of calcium carbonate (CaCO3) as well as other compounds. In an analysis, a chemist takes a sample of limestone which has a mass of 413 mg and treats it with oxalic acid (H2C 2O4). A chemical reaction occurs between the calcium carbonate and the acid producing calcium oxalate an ...
Synthesis of a New Structure B2H4 from B2H6 Highly Selective
... Shown in Fig. 1(a) is the mass spectrum of N-methylformamide excited at the C K-edge. The ratio of mass to charge of the dominant products is 27 – 30 u. To distinguish whether they are products from a specific dissociation, their branching ratios were drawn as in Fig. 2. The products with m/z = 28 u ...
... Shown in Fig. 1(a) is the mass spectrum of N-methylformamide excited at the C K-edge. The ratio of mass to charge of the dominant products is 27 – 30 u. To distinguish whether they are products from a specific dissociation, their branching ratios were drawn as in Fig. 2. The products with m/z = 28 u ...
chapter_2_2007
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
Is Mass Conserved?
... Mass of balloon Mass of 20 ml of baking soda Sum of all reactants before experiment After Experiment Mass of flask, balloon, and products after experiment (when you dump the baking soda into the flask and les the reaction happen, weigh what is left over.) ...
... Mass of balloon Mass of 20 ml of baking soda Sum of all reactants before experiment After Experiment Mass of flask, balloon, and products after experiment (when you dump the baking soda into the flask and les the reaction happen, weigh what is left over.) ...
Organic Chemistry
... spontaneous REDOX reaction Connect two half cells with different electrode potentials A salt bridge connects the two halves ...
... spontaneous REDOX reaction Connect two half cells with different electrode potentials A salt bridge connects the two halves ...
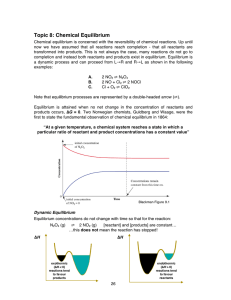
Topic 8: Chemical Equilibrium
... A. Q gets smaller, so the equilibrium shifts to produce more product If concentration increases, the system acts to consume some of it If concentration decreases, the system acts to produce some of it 2. Change in pressure The pressure of a system can be changed in three ways: • Add or remove a gase ...
... A. Q gets smaller, so the equilibrium shifts to produce more product If concentration increases, the system acts to consume some of it If concentration decreases, the system acts to produce some of it 2. Change in pressure The pressure of a system can be changed in three ways: • Add or remove a gase ...
Review Unit - hrsbstaff.ednet.ns.ca
... be broken down into simpler parts by ordinary chemical means. Each element is composed of a fundamental particle called an atom. Each element has a unique atom and is represented by a symbol (memorize the sheet “Some Common Symbols”). ♣ The elements with their symbols are organized into the Periodic ...
... be broken down into simpler parts by ordinary chemical means. Each element is composed of a fundamental particle called an atom. Each element has a unique atom and is represented by a symbol (memorize the sheet “Some Common Symbols”). ♣ The elements with their symbols are organized into the Periodic ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.