word doc (perfect formatting)
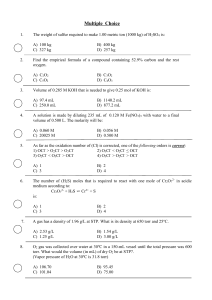
... a) K3PO4 is more soluble in water than KBr b) K3PO4 has a higher molar mass than KBr has. c) To prepare a given volume of 0.10M solution, the mass of K3PO4 needed is more than twice the mass of KBr needed. d) More moles of ions are present in a given volume of 0.10M K3PO4 than in the same volume of ...
... a) K3PO4 is more soluble in water than KBr b) K3PO4 has a higher molar mass than KBr has. c) To prepare a given volume of 0.10M solution, the mass of K3PO4 needed is more than twice the mass of KBr needed. d) More moles of ions are present in a given volume of 0.10M K3PO4 than in the same volume of ...
Final Review 2006
... a. always more than the total mass of the products. b. always less than the total mass of the products. c. sometimes more and sometimes less than the total mass of the products. d. always equal to the total mass of the products. ____ 36. A chemical equation is balanced when the a. coefficients of th ...
... a. always more than the total mass of the products. b. always less than the total mass of the products. c. sometimes more and sometimes less than the total mass of the products. d. always equal to the total mass of the products. ____ 36. A chemical equation is balanced when the a. coefficients of th ...
Slide 1 - Western Engineering
... [ Subscripts: P = product; R = reactant; 0, 1, 2 = temperatures T0, T1 and T2 ] ...
... [ Subscripts: P = product; R = reactant; 0, 1, 2 = temperatures T0, T1 and T2 ] ...
Open questions (66 points total
... 5p 20 Show the relations between the concentrations of the intermediates NH+, NH2+, NH3+ and NH4+ and the concentrations of the reactants: [N+], [H2] and [e]. Use the steady state approximation. 3p 21 Show that the overall production rate of NH3 is given by: ...
... 5p 20 Show the relations between the concentrations of the intermediates NH+, NH2+, NH3+ and NH4+ and the concentrations of the reactants: [N+], [H2] and [e]. Use the steady state approximation. 3p 21 Show that the overall production rate of NH3 is given by: ...
with answers
... Anode: e.g. O2- + C → CO + 2e(f) Most of the sulphur that is needed for the production of sulphuric acid is obtained from the processing of mineral oil or natural gas. Which sulphur-containing compound is obtained in this way? Which process is used to obtain sulphur from this compound? Give the reac ...
... Anode: e.g. O2- + C → CO + 2e(f) Most of the sulphur that is needed for the production of sulphuric acid is obtained from the processing of mineral oil or natural gas. Which sulphur-containing compound is obtained in this way? Which process is used to obtain sulphur from this compound? Give the reac ...
Chemistry I Syllabus 2011-2012
... added to or removed from the system. Elements and compounds undergo changes of state and re-arrangements of atoms that accompany changes in energy. Their ...
... added to or removed from the system. Elements and compounds undergo changes of state and re-arrangements of atoms that accompany changes in energy. Their ...
chapter 5 lecture notes ppt
... system is independent of the path by which the system achieved that state. In the system below, the water could have reached room temperature from either direction. ...
... system is independent of the path by which the system achieved that state. In the system below, the water could have reached room temperature from either direction. ...
Examination
... Carbon dioxide, CO2, changes from the solid phase to the gas phase at 1 atm and 194.5 K. In the solid phase, CO2 is often called dry ice. When dry ice sublimes in air at 298 K, the water vapor in the air can condense, forming a fog of small water droplets. This fog is often used for special effects ...
... Carbon dioxide, CO2, changes from the solid phase to the gas phase at 1 atm and 194.5 K. In the solid phase, CO2 is often called dry ice. When dry ice sublimes in air at 298 K, the water vapor in the air can condense, forming a fog of small water droplets. This fog is often used for special effects ...
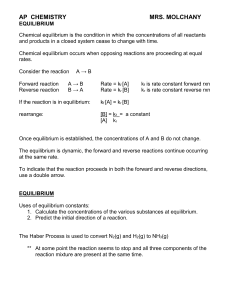
Chemical Equilibrium - Shailendra Kumar Chemistry
... concentration of C at equilibrium is increased by a factor 2, it will cause the equilibrium concentration of B to change to: (a) two times of its original value (b) one half of its original value (c) 2√2 times of its original value (d) 1/2√2 times of its original value ...
... concentration of C at equilibrium is increased by a factor 2, it will cause the equilibrium concentration of B to change to: (a) two times of its original value (b) one half of its original value (c) 2√2 times of its original value (d) 1/2√2 times of its original value ...
equilibrium - TeacherWeb
... 3. Use the stoichiometry of the reaction (this is, use the coefficients in the balanced chemical equation) to calculate the changes in concentration for all the other species in the equilibrium. 4. From the initial concentrations and the changes in concentration, calculate the equilibrium concentrat ...
... 3. Use the stoichiometry of the reaction (this is, use the coefficients in the balanced chemical equation) to calculate the changes in concentration for all the other species in the equilibrium. 4. From the initial concentrations and the changes in concentration, calculate the equilibrium concentrat ...
2013 us national chemistry olympiad
... 5. [12] Write net equations for each of the reactions below. Use ionic and molecular formulas as appropriate and omit formulas for all ions or molecules that do not take part in a reaction. Write structural formulas for all organic substances. You need not balance the equations or specify physical ...
... 5. [12] Write net equations for each of the reactions below. Use ionic and molecular formulas as appropriate and omit formulas for all ions or molecules that do not take part in a reaction. Write structural formulas for all organic substances. You need not balance the equations or specify physical ...
Gas-Forming reactions Reactions that form a
... But they end up with the same number of electrons they start with. Every atom, ion or polyatomic ion has a formal oxidation number associated with it. This value compares the number of protons in an atom (positive charge) and the number of electrons assigned to that atom (negative charge). In many c ...
... But they end up with the same number of electrons they start with. Every atom, ion or polyatomic ion has a formal oxidation number associated with it. This value compares the number of protons in an atom (positive charge) and the number of electrons assigned to that atom (negative charge). In many c ...
Name: Northwest Vista College Chem 1311
... Reaction B: Products are at a higher energy content than reactants. 250 kJ are required to activate the reaction. A total of 100 kJ are absorbed by the reaction. It is endothermic. 2. Choose one of the hypothetical reactions in the diagrams above and write a general thermochemical equation for it us ...
... Reaction B: Products are at a higher energy content than reactants. 250 kJ are required to activate the reaction. A total of 100 kJ are absorbed by the reaction. It is endothermic. 2. Choose one of the hypothetical reactions in the diagrams above and write a general thermochemical equation for it us ...
Document
... • Selection rules arise from the resonance condition, which may be expressed as a transition dipole moment: ...
... • Selection rules arise from the resonance condition, which may be expressed as a transition dipole moment: ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.