Chapter 6 Chemical reactions Classification And Mass Relationships
... The chemical reaction is represented by a chemical equation in which the chemicals present before the reaction (reactants) are written on the left and the chemicals formed after the reaction (products) are written on the right of an arrow that represents the direction of the chemical reaction. Reac ...
... The chemical reaction is represented by a chemical equation in which the chemicals present before the reaction (reactants) are written on the left and the chemicals formed after the reaction (products) are written on the right of an arrow that represents the direction of the chemical reaction. Reac ...
Name: 1) What is the oxidation number of sulfur in H SO ? A)
... 67) The diagram below represents the electroplating of a metal fork with Ag(s). ...
... 67) The diagram below represents the electroplating of a metal fork with Ag(s). ...
Chemical Reactions
... To write a word equation, write the names of the reactants to the left of the arrow separated by plus signs. Write the names of the products to the right of the arrow, also separated by plus ...
... To write a word equation, write the names of the reactants to the left of the arrow separated by plus signs. Write the names of the products to the right of the arrow, also separated by plus ...
Chemistry Name Mr. Reger Review Guide – Ch. 9
... 3 Cl2(g) + 6 NaOH(aq) 5 NaCl(aq) + NaClO3(aq) + 3 H2O(l) 11. Use the equation in the question above to answer the following: a) What is the theoretical yield of NaClO3 if 4.0mol Cl2 is reacted with excess NaOH? b) If 94.2g NaClO3 is obtained, what is the % yield? c) A different student performs th ...
... 3 Cl2(g) + 6 NaOH(aq) 5 NaCl(aq) + NaClO3(aq) + 3 H2O(l) 11. Use the equation in the question above to answer the following: a) What is the theoretical yield of NaClO3 if 4.0mol Cl2 is reacted with excess NaOH? b) If 94.2g NaClO3 is obtained, what is the % yield? c) A different student performs th ...
Specific Reactions Quiz.wpd
... a) various carbon products created due to lack of oxygen including solid carbon (black component) b) as air contacts the random carbon products (smaller hydrocarbons) created, they may further combust c) since energy is still tied up in carbon product bonds, energy is not released all at once d) the ...
... a) various carbon products created due to lack of oxygen including solid carbon (black component) b) as air contacts the random carbon products (smaller hydrocarbons) created, they may further combust c) since energy is still tied up in carbon product bonds, energy is not released all at once d) the ...
Transition State Theory
... with the reaction coordinate given in terms of the distance between the B and C molecules. The reaction coordinate for this reaction was discussed in R.3-A Collision Theory–D Polyani Equations when discussing the Polanyi equation. † barrier shown in Figure R3.B-2 is the shallowest barrier The energy ...
... with the reaction coordinate given in terms of the distance between the B and C molecules. The reaction coordinate for this reaction was discussed in R.3-A Collision Theory–D Polyani Equations when discussing the Polanyi equation. † barrier shown in Figure R3.B-2 is the shallowest barrier The energy ...
as a PDF
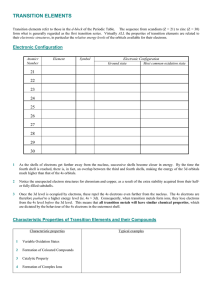
... Both ligand field effects and inter-electronic repulsion produce irregularities in the chemistry of transition series. Irregularities due to inter-electronic repulsion are most obvious in the lanthanide series where ligand field effects are very small. For the first century of lanthanide chemistry, ...
... Both ligand field effects and inter-electronic repulsion produce irregularities in the chemistry of transition series. Irregularities due to inter-electronic repulsion are most obvious in the lanthanide series where ligand field effects are very small. For the first century of lanthanide chemistry, ...
Chapter 17 Notes
... SI unit for energy - joule (J) (capital - named for James P. Joule - British) The Law of Conservation of Energy: energy can be converted from one form to another. example - food is chemical energy - converted to mechanical - limb motion example - light energy enters our eyes - converted to electrica ...
... SI unit for energy - joule (J) (capital - named for James P. Joule - British) The Law of Conservation of Energy: energy can be converted from one form to another. example - food is chemical energy - converted to mechanical - limb motion example - light energy enters our eyes - converted to electrica ...
Qualitative Analysis Lab
... can be classified as a combustion reaction because it depicts the burning of carbon, and it can be classified as a combination reaction because two reactants combine to give one product. It can also be classified as an oxidation reaction because carbon is oxidized during the reaction. It is also a r ...
... can be classified as a combustion reaction because it depicts the burning of carbon, and it can be classified as a combination reaction because two reactants combine to give one product. It can also be classified as an oxidation reaction because carbon is oxidized during the reaction. It is also a r ...
Name
... How do you determine the number of valence electrons for an element using the periodic table? Give the number of valence electrons for: ...
... How do you determine the number of valence electrons for an element using the periodic table? Give the number of valence electrons for: ...
Precipitation and Redox Reactions
... • Hydrogen peroxide also releases oxygen when it decomposes 2 H2O2 (aq) 2 H2O (l) + O2 (g) ...
... • Hydrogen peroxide also releases oxygen when it decomposes 2 H2O2 (aq) 2 H2O (l) + O2 (g) ...
Introductory Chemistry: A Foundation FOURTH EDITION by Steven
... has mass and volume • Energy is the part of the universe that has the ability to do work • Chemistry is the study of matter – The properties of different types of matter – The way matter behaves when influenced by other matter and/or energy ...
... has mass and volume • Energy is the part of the universe that has the ability to do work • Chemistry is the study of matter – The properties of different types of matter – The way matter behaves when influenced by other matter and/or energy ...
Student Review packet
... Suppose the substances in the reaction above are at equilibrium at 600 K in volume V and at pressure P. State whether the partial pressure of NH3(g) will have increased, decreased, or remained the same when equilibrium is reestablished after each of the following disturbances of the original system. ...
... Suppose the substances in the reaction above are at equilibrium at 600 K in volume V and at pressure P. State whether the partial pressure of NH3(g) will have increased, decreased, or remained the same when equilibrium is reestablished after each of the following disturbances of the original system. ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.